The COVID 19 vaccination drive in Pakistan has already been launched. People including healthcare personnel are unsure of the vaccination efficacy and hesitant to get vaccinated.
For public awareness, I am discussing key points here that might answer some of the vaccination-related queries.
Covid 19 Vaccines approved in Pakistan:
Currently, three vaccines have been approved for EUA (emergency use authorization) in Pakistan:
- Sinopharm
- Astrazeneca by Oxford
- Sputnik by Russia
While two candidate Vaccines are conducting phase 3 clinical trials:
- CanSino ( NIH, UHS, AKUH, SIH)
- RBD Dimer (UHS, AKUH) Both by China
A brief overview of the COVID 19 Vaccines approved for EUA in Pakistan:
Sinpharm Vaccine (Sinopharm BBIBP-CORV Vaccine):
Sinopharm Vaccine (Sinopharm BBIBP-CORV Vaccine) has been derived from SARS-CoV-2 infected individuals in China.
It is an inactivated Vaccine (Aluminum hydroxide adjuvant) that has to be administered intramuscularly in two doses, 28 days apart. It requires a storage temperature of 2 – 8 C.
Sinopharm Vaccine has an efficacy of 79% (86% in the United Arab Emirates)
It is also approved in China, Bahrain, Egypt, and Jordan and is currently being given to frontline healthcare workers in Pakistan in the initial phase.
It has minimal side effects. Local side effects include redness, pain, itching, and swelling. Systemic side effects include fever, itching, fatigue, body aches, flu, and diarrhea.
Sinopharm Vaccine efficacy: 79 – 86%
AstraZeneca Vaccine:
It is an adenovirus-derived vaccine with an overall efficacy of around 70%. Clinical trials from Brazil and UK demonstrated the efficacy of around 90%. Two doses, 28 days apart should be administered intramuscularly.
It is also approved in UK, Argentina, India, and Mexico. Side effects of the drugs include fever, headache, myalgias, and fatigue (about 8% of the vaccinated population). Two cases of transverse myelitis have been associated with its use. One case was possibly associated with the vaccine while the other case was thought to have been present before the vaccine and identified later.
AstraZeneca Vaccine Efficacy: 70 – 90%
SPUTNIK V Covid Vaccine:
The vaccine was developed by Russia’s National Research Center for Epidemiology and Microbiology. It has demonstrated the efficacy of around 92% and will be potentially sold in India, Korea, Brazil, China, and Hungary with costs of about $10 per dose.
It is an adenovirus-derived vaccine. Two doses of the vaccines are required.
Sputnik Vaccine Efficacy: 92%
CanSino Biologics:
Cansino Biologic vaccine was developed by the Chinese military. It is an adenovirus-based vaccine that has an efficacy of 65% in preventing symptomatic cases and 90% in preventing severe disease. Currently, it is undergoing a multi-country analysis. NIH, UHS, AKUH, and SIH are part of the study. It is also approved by WHO as a part of the COVAX program.
A single dose of the vaccine is recommended.
CanSino Biologic Vaccine Efficacy: 65 – 90%
RBD Dimer:
It is a viral protein-based vaccine that is currently undergoing phase 3 trials in Pakistan, Uzbekistan, and Malaysia. UHS and AKUH are part of the study project.
Three doses of the vaccine are recommended. The efficacy of the vaccines is not known yet.
RBD Dimer Vaccine efficacy: Not known yet
What does COVID-19 Vaccines efficacy mean?
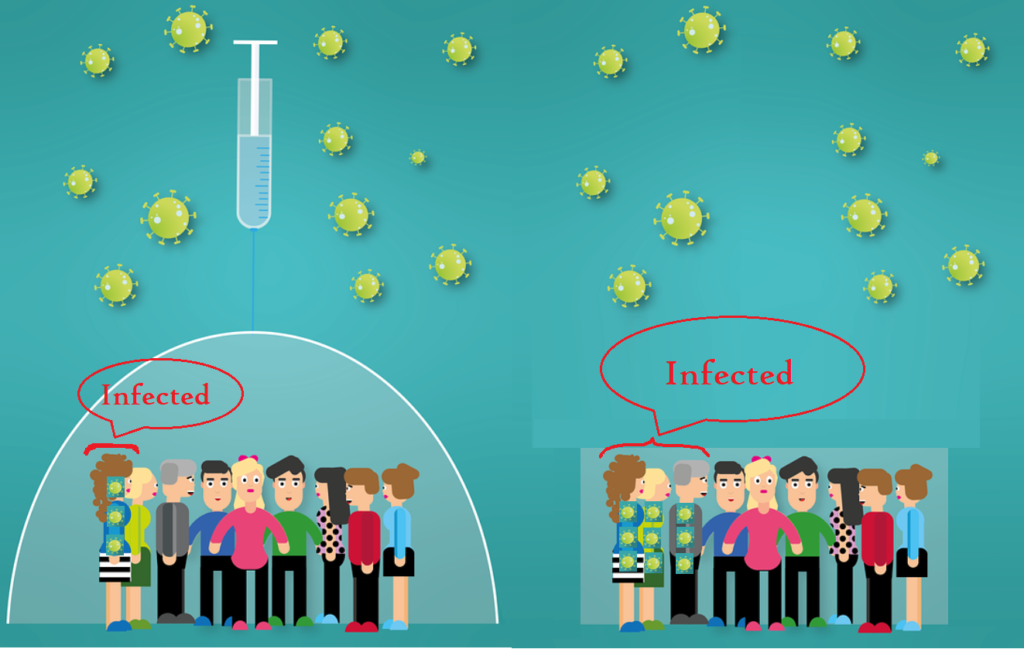
The efficacy of the above vaccines has been demonstrated to be around 70% or 90%. What does 70% efficacy mean?
Does it mean that if you vaccinate 100,000 individuals,
it will protect 70% (70,000) individuals and the rest of the 30,000 (30% of the Vaccinated individuals) will get the disease?
Or,
Vaccine efficacy of 90% means 90% (90,000) will be protected and 10,000 of the individuals will get the disease.
No.
The vaccine efficacy in clinical trials is estimated by the formula:
100 x (1 – Covid Infection Rate in Vaccinated patients/ Covid Infection Rate in Unvaccinated Cases)
Let’s suppose the vaccine is 70% effective, what will be the Covid 19 infection rate in vaccinated individuals:
To estimate the number of vaccinated individuals who will get the disease, first, we should know the rates of infection in the unvaccinated population.
Currently, in Pakistan, the infectivity rate is estimated to be 3.9% to 4.8%. This means in a population sample size of 100,000, 3900 to 4800 people get infected.
With a vaccine efficacy of 70%, the number of individuals who will get the disease:
100 x (1 – infectivity rate in vaccinated/ infectivity rate in unvaccinated) = 70% efficacy
We have to find the infectivity rate in the unvaccinated individuals. So this is equal to:
= 100 x (1 – infectivity rate in the vaccinated/ 3900) = 70%
= infectivity rate = 1170 or 1.17%
The infectivity rate will drop from 3.9% to 1.17% with a vaccine efficacy of 70% and an infectivity rate of 3.9%.
Similarly, with a vaccine efficacy of 90% and an infectivity rate of 3.9% in a population, the infectivity rate will drop to 390 or 0.39% compared to 3900 or 3.9% in the unvaccinated individuals.
So, 90% efficacy does not mean that 10% get the infection, only 0.39% get the infection
The first covid vaccine shipment containing half a million SINOPHARM vaccines has arrived in Pakistan, further, we have also secured 17 million indicative doses of the ASTRAZENECA vaccine, out of which 35-40 percent or 6 to 6.8 million doses will be available within the first quarter of the current year.
Read details of all the vaccines here: covid-19-vaccination overview.