Diabetes Mellitus, an incurable disease, is known to be a silent killer. Most of the complications of diabetes and deaths result from advancing disease status and progressing target end-organ damage.
Diabetes is not just about numbers but preventing one’s self from acquiring the dreadful Complications of diabetes mellitus.
People who are compliant to the diabetic diet and medicines, exercise regularly and control their blood sugars are less likely to get the complications of diabetes.
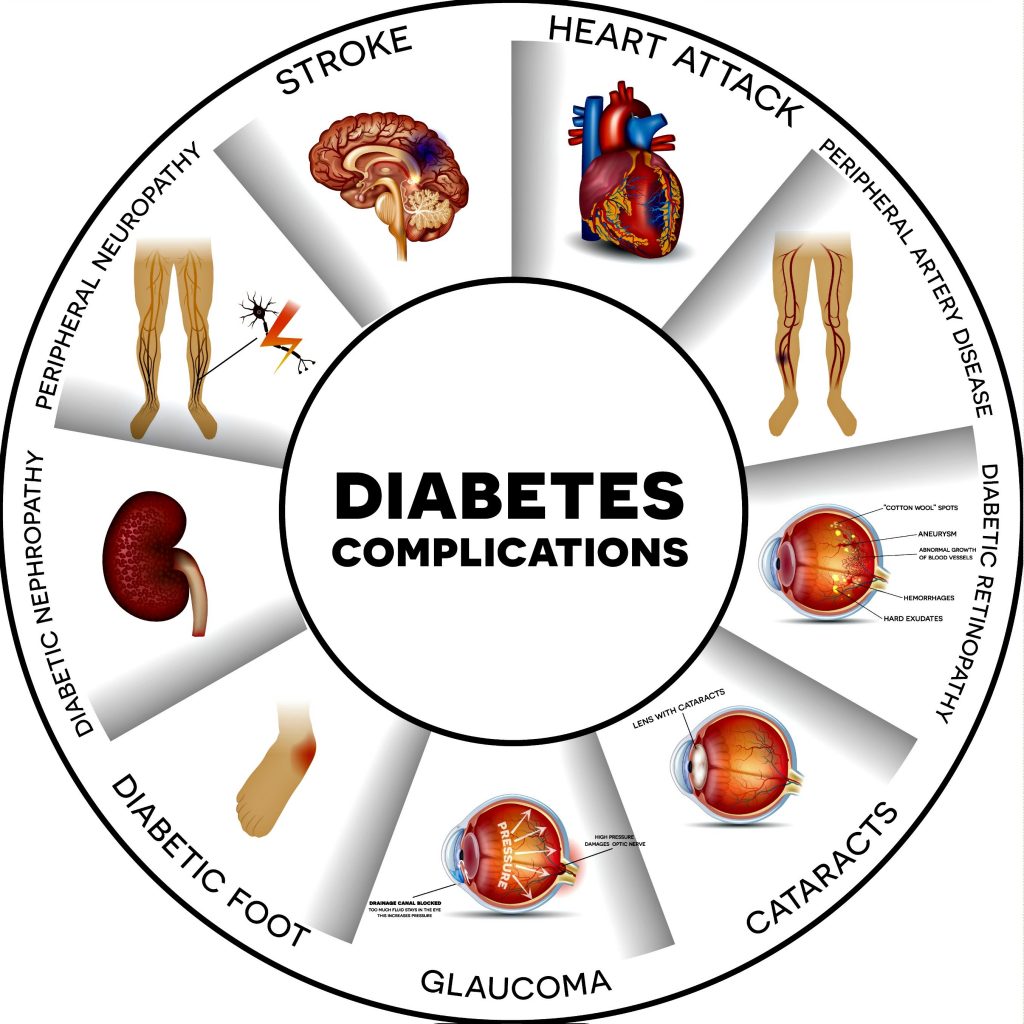
Complications of Diabetes Mellitus can be divided into acute and chronic.
Acute complications of diabetes:
Diabetic ketoacidosis (DKA) and hyperosmolar hyperglycemic states (HHS) are acute complications of diabetes. Previously DKA was considered as the hallmark of T1DM but it can also occur in patients with T2DM.

HHS is primarily seen in patients with T2DM.
Treatment of both diseases is the same as both are associated with relative or absolute insulin deficiency, volume depletion, and acid-base disorder.
Both conditions can be fatal if not promptly recognized and treated.
Diabetic Ketoacidosis:
Diabetic Ketoacidosis (DKA) is characterized by hyperglycemia, volume depletion, non-anion gap metabolic acidosis, and ketosis.
It predominantly occurs in T1DM but can also occur in T2DM. The usual cause is non-compliance to treatment and diet, underlying infection, ischemia especially in the elderly and certain medications.

Hyperglycemia results in glucosuria, volume depletion, hypotension, and tachycardia.
Metabolic acidosis can result in Kussmaul respiration, acetone breath, abdominal pain, vomiting, central nervous system, and cardiovascular depression.
Patients may present in a comatose state which may be due to DKA but other causes of altered sensorium like ischemia, infection, and hypoxemia should always be ruled out.
Diagnosis and treatment of DKA:
Prompt diagnosis of DKA is important as it is associated with significant morbidity and mortality if untreated.
Diagnosis requires the evidence of hyperglycemia, anion gap metabolic acidosis, and ketosis. Patients are usually dehydrated. Sodium levels may be falsely low owing to hyperglycemia.
Potassium levels may be normal despite a total body potassium deficit. Amylase may be raised so pancreatitis must be ruled out by measuring lipase levels. Reasons for patients going into ketosis must be looked at.
Treatment is directed at replacing adequate intravenous IV fluids and electrolytes, insulin therapy and identifying and treating the precipitant.
Patients should be monitored to ensure improvement. Intravenous insulin should be continued until acidosis resolves and the patient is metabolically stable.
Bicarbonate replacement is usually not necessary but is advised if arterial PH drops to 6.9 or less.
Stress ulcer and DVT prophylaxis should be given to all patients unless contraindicated as venous thrombosis, upper gastrointestinal bleeding, and acute respiratory distress syndrome occasionally complicate DKA.
Cerebral edema is also a known complication especially when hyperglycemia is rapidly corrected.
Patients should be educated about adequate fluid intake, noncompliance issues and prompt treatment of concurrent illnesses.
Hyperglycemic Hyperosmolar State (HHS):
Hyperglycemic Hyperosmolar state usually occurs in T2DM, elderly patients who have hyperglycemia lasting for days to weeks associated with vomiting, reduced intake, weight loss, confusion and finally coma.
The estimated mortality in HHS is about ten times higher than in diabetic ketoacidosis. Patients have profound hyperglycemia, hypotension, tachycardia, and hyperosmolality.

HHS is often precipitated by stroke, myocardial infarction, sepsis, pneumonia, and other serious infection. Hyperglycemia leads to osmotic diuresis causing dehydration and intravascular volume depletion. Acidosis and ketosis are not present usually, probably due to relative insulin deficiency which is less severe than in DKA.
Laboratory features in HHS include marked hyperglycemia, hyperosmolality and prerenal azotemia. Serum sodium can be falsely low due to hyperglycemia.
Treatment of HHS:
Treatment of DKA and HHS is essentially the same but patients with HHS are usually older, have more serious comorbidities and so have high mortality.
The fluid requirement is also high in these patients but replacement of fluid must be balanced according to the patients need.
Rapid reversal of hyperglycemia and volume status may worsen neurologic function. Hypokalemia, hypomagnesemia, and hypophosphatemia should be corrected especially in patients taking diuretics.
Chronic Complications of Diabetes Mellitus:

Chronic complications of diabetes mellitus are responsible for most of the morbidities and mortalities. Chronic complications can be either vascular or nonvascular.
Vascular complications can be microvascular ( retinopathy, neuropathy, nephropathy) and macrovascular ( coronary heart disease, peripheral arterial disease, and cerebrovascular disease).
Nonvascular complications include infections, skin changes, and gastroparesis.
Microvascular complications have been associated with chronic hyperglycemia while there is little evidence correlating macrovascular complications and hyperglycemia.
Glycemic control and complications:
Reduction in hyperglycemia has been proven to prevent chronic complications of diabetes especially microvascular complications (retinopathy, nephropathy, and neuropathy).
Reduction in macrovascular complications has not been shown to be significantly reduced. In the UKPDS trial, 35% reduction in microvascular complications was noted when HbA1C was lowered by a single percentage point.
The DCCT, UKPDS and a Japanese trial (Kumamoto study) all emphasize intensive glycemic control in all forms of DM and early diagnosis and strict blood pressure control in Type 2 DM.
Ophthalmologic complications of Diabetes Mellitus:
Diabetes is the leading cause of blindness worldwide. Blindness is primarily the result of diabetic retinopathy and clinically significant macular edema.

Diabetic retinopathy is classified into two stages:
- Nonproliferative and
- Proliferative.
Nonproliferative retinopathy usually appears in the early second decade of the disease and is characterized by retinal microaneurysms, blot hemorrhages, and cotton wool spots.
Neovascularization is the hallmark of proliferative diabetic retinopathy. These newly formed vessels rupture easily leading to vitreous hemorrhages, fibrosis and ultimately retinal detachment.
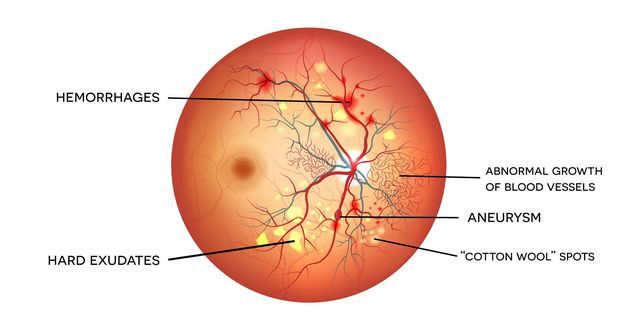
Uncontrolled hyperglycemia for a prolonged duration is a strong predictor of the development of retinopathy. Prevention is the most important strategy for diabetic retinopathy.
Intensive glycemic and blood pressure control will delay the development or progression of diabetic retinopathy.

Paradoxically in the first 6 to 12 months of improved glycemic control, the retinopathy worsens but this progression is temporary.
Prophylactic photocoagulation is advised in patients with known diabetic retinopathy when initiating intensive therapy.
Adequate glycemic control is less effective when there is advanced retinopathy.
Proliferative retinopathy is treated with pan-retinal laser photocoagulation whereas macular edema is treated with focal photocoagulation.
Renal complications of diabetes mellitus:
Diabetic nephropathy is the leading cause of diabetes-related morbidity and mortality worldwide.
Patients with diabetic nephropathy commonly have diabetic retinopathy. Renal hypertrophy and glomerular hyperperfusion occur early followed by glomerular basement membrane thickening, glomerular hypertrophy and mesangial volume expansion as the GFR returns to normal.
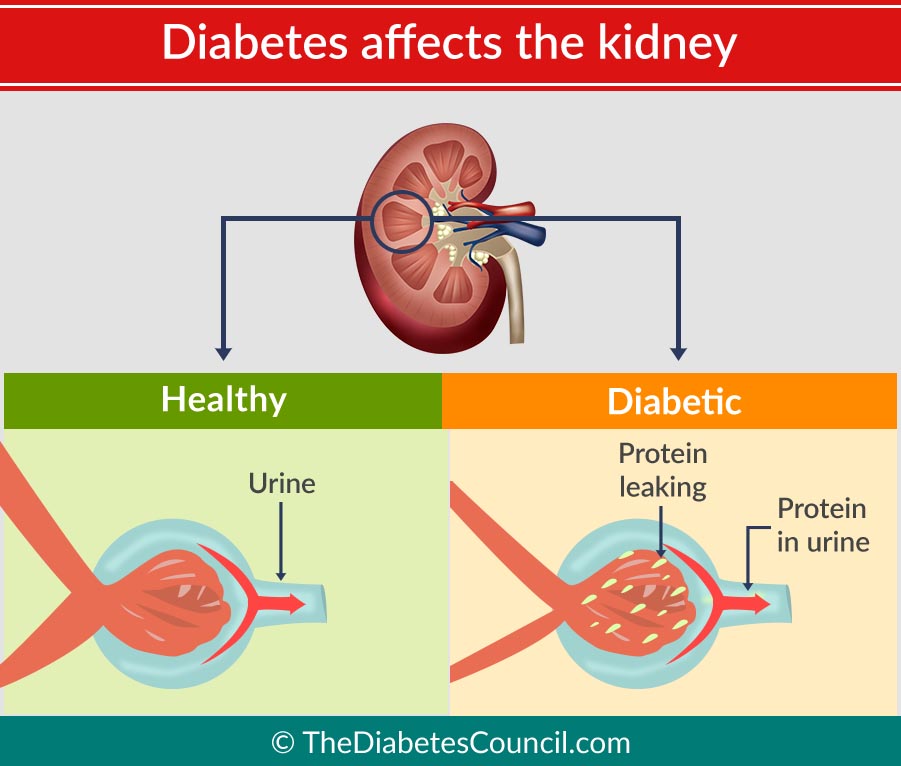
Microalbuminuria, defined as 30 – 299 mg/day in a 24-hour collection or 30 – 299 ug/mg creatinine in a spot collection, develops in about 40% of T1DM patients after ten years of the disease. About half of the patients will progress to macroalbuminuria (>300 mg/day or > 300 ug/mg creatinine) in the next ten years.
Microalbuminuria is a risk factor for cardiovascular disease. In 7-10 years about half of the patients will progress to ESRD (end-stage renal disease).
Changes developing in the kidney after macroalbuminuria develops are not reversible. Type IV renal tubular acidosis ( hyporeninemic hypoaldosteronism) may occur in both type 1 or 2 DM.
Patients with diabetes are predisposed to contrast-induced nephrotoxicity especially in hypovolemic states and preexisting nephropathy.
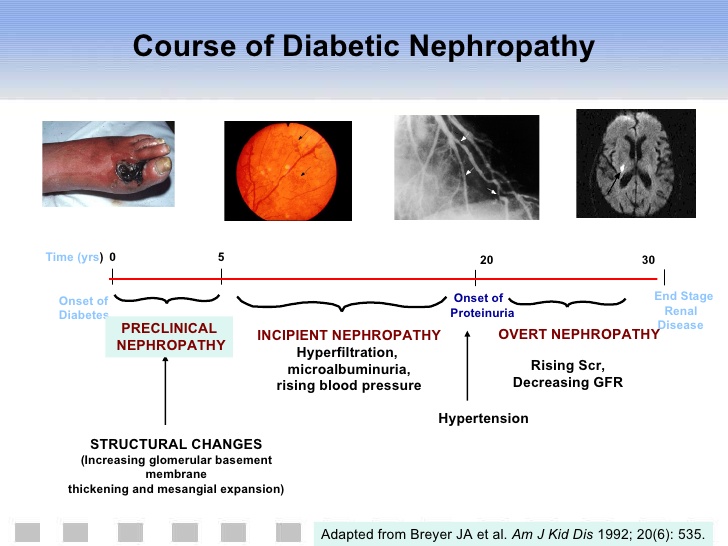
The optimal therapy for diabetic nephropathy is prevention and euglycemia.
Microalbuminuria and annual measurement of serum creatinine to estimate GFR should be performed for early detection at an early stage.
Interventions which delay the progression of diabetic nephropathy should be instituted early. These include euglycemic state, strict blood pressure control, administration of ACE inhibitors or ARBs and treating dyslipidemia.
As the kidney status declines insulin requirements fall. Sulfonylureas and metformin are contraindicated in advanced renal insufficiency.
Hypertension develops in many patients with diabetic nephropathy, especially with advanced renal dysfunction.
ACE inhibitors and ARBs are effective in reducing albumin excretion and slowing the decline in renal function.
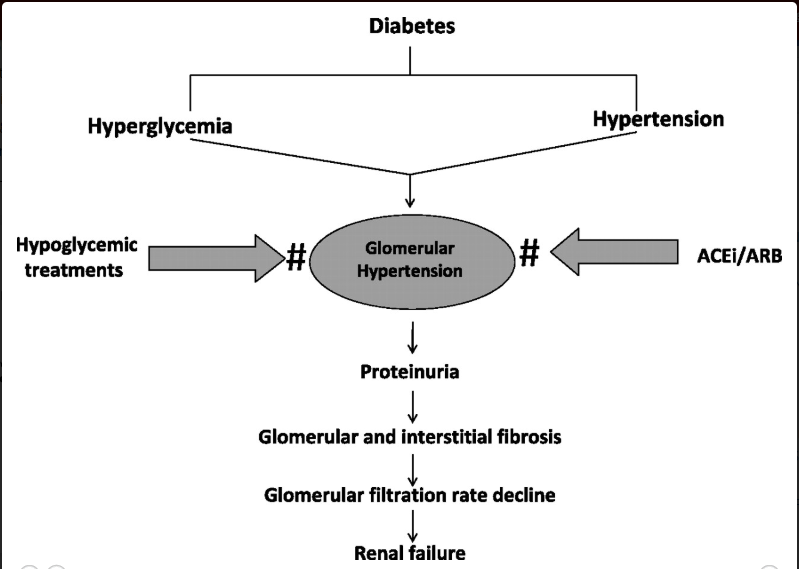
Blood pressure should be maintained at a target BP of < 130/80 mmHg. The dose of ACE inhibitors and ARBs is increased at 2 -3 months interval if microalbuminuria persists.
Another class of drug may be used preferably calcium channel blockers (non-dihydropyridine class) if target BP is not achieved with ACE inhibitors or ARBs or are not tolerated.
The ADA suggests modest restriction of protein intake in diabetic individuals with microalbuminuria ( 0.8 – 1.0 gm/kg/day) or macroalbuminuria ( < 0.8 gm/kg/day). If GFR falls to less than 60 ml/min/1.743 m2, consultation with a nephrologist should be done.
Atherosclerosis is the leading cause of death in these patients. Renal transplantation from a living donor is the preferred treatment.
Neuropathy and Diabetes Mellitus:
Diabetic neuropathy occurs frequently in individuals with longstanding diabetes. It may manifest as mononeuropathy, polyneuropathy or autonomic neuropathy.
Risk factors for the development of diabetic neuropathy are long-standing diabetes, glycemic control, obesity, and smoking.
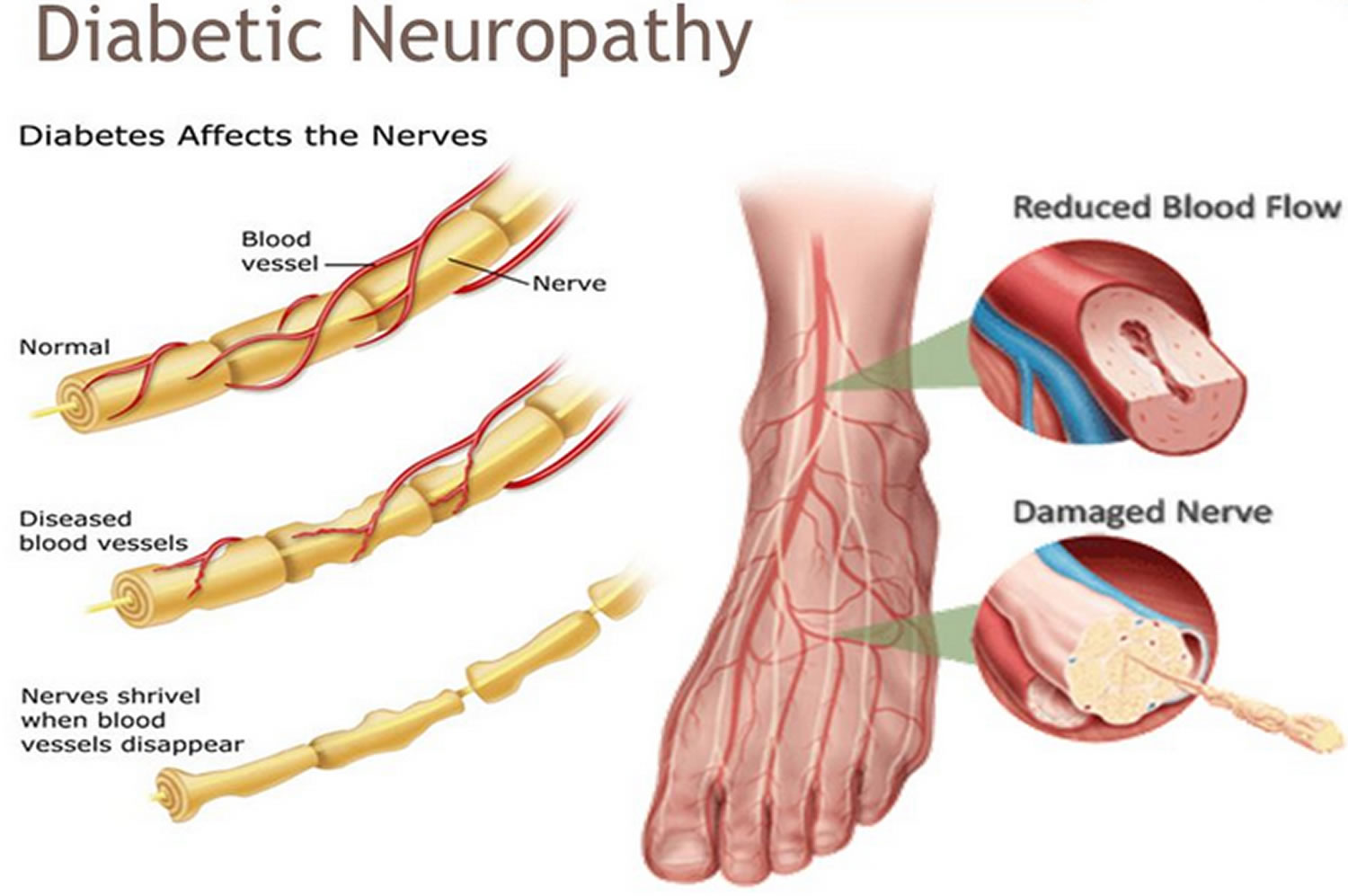
Polyneuropathy/ mononeuropathy: distal symmetric polyneuropathy is the most common form of diabetic neuropathy.
It may present as hyperesthesia, paresthesia, and dysesthesia but most frequently presents with a distal sensory loss.
Neuropathic pain commonly involves the lower limbs and worsens at night. With disease, progression pain disappears but sensory deficit persists.
Physical examination reveals sensory loss, absent ankle reflexes, and abnormal position sense.

Diabetic polyradiculopathy is characterized by severe disabling pain in the distribution of one or more nerve roots accompanied by motor weakness.
Diabetic polyradiculopathies resolve over a period of 6 to 12 months.
Mononeuropathy is less common than polyneuropathy and presents with pain and motor weakness in the distribution of a single nerve.
The 3rd cranial nerve is the most commonly involved and patients present with diplopia, ptosis, ophthalmoplegia with normal pupillary constriction to light.
Peripheral mononeuropathies and mono neuritis multiplex are also not uncommon.
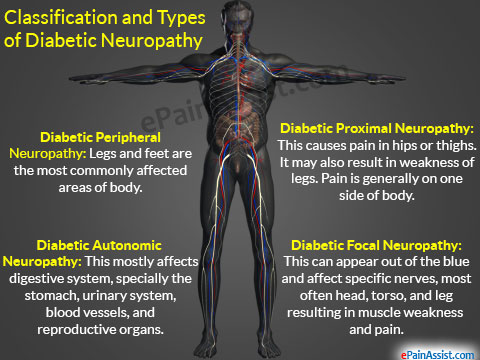
Autonomic neuropathy: diabetic autonomic neuropathy can involve various organ systems. Dysautonomia can result in resting tachycardia, orthostatic hypotension, and rarely sudden death.
Gastroparesis, bladder emptying disorders, hyperhidrosis (excessive sweating) of the upper extremities and anhidrosis (reduce/ absent sweating) of the lower limbs ( sympathetic nervous system dysfunction).
Autonomic neuropathy may also lead to an inability to sense hypoglycemic symptoms and increases the risk of diabetic foot ulcers secondary to anhidrosis and dry feet.
Treatment of Diabetic neuropathy:
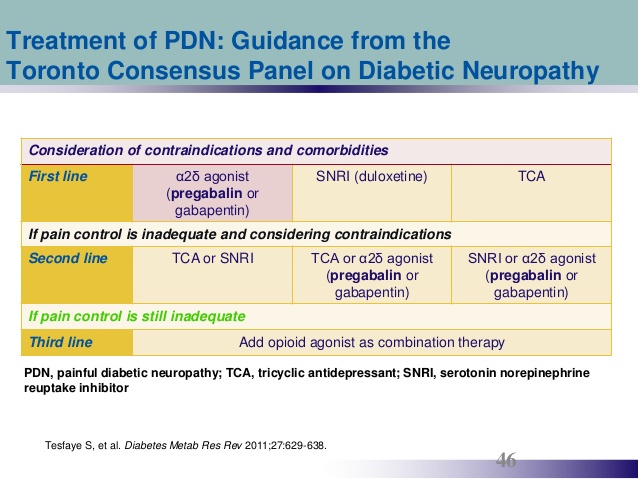
Treatment of diabetic neuropathy involves glycemic control, treating and avoiding risk factors like hypertension, hypertriglyceridemia, symptomatic treatment and supplementations with vitamins (B12, folate).
Frequent feet examinations and proper precautions (footwear) aimed at preventing calluses or ulcerations should be taken.
Antidepressants ( tricyclic antidepressants and selective serotonin-norepinephrine reuptake inhibitors) or anticonvulsants may be used to treat chronic painful diabetic neuropathy. Orthostatic hypotension is also difficult to treat.
A variety of agents can be used including fludrocortisone, midodrine, clonidine, and octreotide.
Gastrointestinal and genitourinary complaints:
Gastroparesis and altered small and large bowel motility are the most common GI manifestations.
Metoclopramide, domperidone, and erythromycin may be used for gastroparesis. Nocturnal diarrhea with alternating constipation is a feature of DM-related GI autonomic neuropathy. Loperamide and octreotide may be used for diarrhea in these patients.
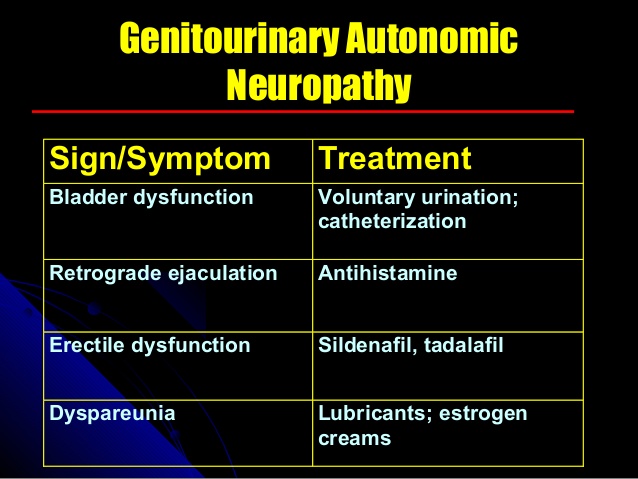
Cystopathy, erectile dysfunction, and female sexual dysfunction are genitourinary manifestations of diabetic autonomic neuropathy.
Diabetic cystopathy should be treated with timed voiding or self-catheterization and possibly bethanechol.
Phosphodiesterase inhibitors may be used for erectile dysfunction in men. Vaginal lubricants and systemic or topical estrogens may be used in women.
Cardiovascular morbidity and mortality:
Type 2 Diabetes has been labeled as coronary heart disease equivalent. There is a marked increase in peripheral arterial disease, coronary heart disease, congestive heart failure, myocardial infarction, and sudden death.
Silent ischemia due to neuropathy may occur in diabetics. Therefore diabetic patients should be properly screened especially preoperatively.
Other risk factors ( dyslipidemia, smoking cessation, hypertension, obesity) for ischemic heart disease should be aggressively managed.
Reduction in cardiovascular morbidity and mortality has been shown in studies with improved glycemic control only after a follow up of 10 to 17 years.
Treatment of coronary disease in diabetic patients is not different from the general population but revascularization procedures are generally less efficacious and chances of restenosis are very high.
Glycemic control and aggressive cardiovascular risk modification have been emphasized by ADA. Aspirin should be used in every diabetic patient who has risk factors for CHD. Beta-blockers and ACE inhibitors may benefit diabetic patients if indicated.
Lower extremity complications:
Various pathogenic factors leading to diabetic foot ulcers, infections and ultimately amputations are involved.
These factors include neuropathy, abnormal foot biomechanics, PAD, and poor wound healing.
About 15% diabetic individuals develop a foot ulcer and a significant subset will ultimately undergo amputation.
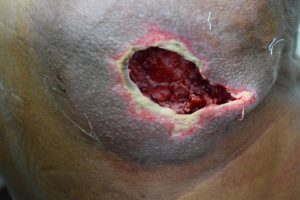
Treatment for foot ulcers is through education, measures to prevent ulceration, routine foot examination, identification of high-risk patients, evaluation by a foot care specialist, optimizing other risk factors like dyslipidemia and hypertension and a good glycemic control.
The plantar surface of the foot is the most common site of ulceration. Ulcers may be neuropathic, ischemic or neuro-ischemic.
When there is accompanying cellulitis or osteomyelitis, antibiotics may be required. Culture should be taken from the wound base or discharging purulent material.
Ulcers that do not heal should be investigated for underlying osteomyelitis via simple radiography, bone scans or MRI. Six interventions have been recognized by ADA as effective modalities in the treatment of diabetic ulcers. These include:
- off-loading,
- debridement,
- wound dressings,
- appropriate use of antibiotics,
- revascularization, and
- limited amputation
Infections:
Diabetic individuals have a greater frequency and severity of infections. Chronic hyperglycemia leads to colonization of common pathogens like candida whereas several rare pathogens are seen almost exclusively in diabetic individuals.
These include rhino-cerebral mucormycosis, emphysematous infections of the gallbladder and urinary tract and malignant otitis externa. Postoperative wound infections are also more common in diabetic individuals, especially in uncontrolled glycemic status.
Dermatologic manifestations:
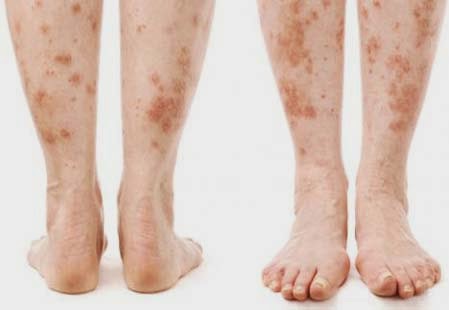
Skin ulcerations and protracted wound healing are the most common skin manifestations.
Diabetic dermopathy (pigmented pretibial papules or diabetic skin spots) begins as erythematous area and evolves into an area of hyperpigmentation.
Bullous diseases such as bullosa diabeticorum are also seen.
Necrobiosis lipoidica diabeticorum is usually seen in young women with type 1 DM, neuropathy and retinopathy.
Vitiligo occurs at increased frequency in type 1 DM. acanthosis nigricans a feature of insulin resistance is sometimes seen.
Granuloma annulare and scleredema are more common in diabetics. Lipoatrophy and lipohypertrophy can occur at sites of insulin injections.
Xerosis and pruritis are common and relieved by moisturizers.
Read More …