I have already published the WHO updated vaccination schedule for children. Since vaccination in adults is often neglected, this article focuses on the updated CDC vaccine schedule for adults.
Vaccination should be an integral part of the health affairs of a country. The primary focus of all the vaccination campaigns is revolving around children. This has led to the misconception that vaccination in adults is probably not essential.
Contrary to this belief, vaccination in adults is equally important and should be emphasized especially in a certain group of patients. Most childhood diseases when affecting the adults can be very severe and may be fatal.
A few examples of vaccine-preventable diseases in adults
- Varicella infection does not require treatment in a child less than 12 years of age but should always be treated in adults and immunocompromised individuals. Untreated adults are at risk of developing varicella pneumonia and encephalitis.
- Similarly, mumps in children can be a self-resolving disease but in adults, it leads to mumps orchitis, pancreatitis and other organ dysfunction in adults frequently.
- Similarly, HPV vaccination in early adulthood can prevent cervical cancer.
Table of contents – CDC vaccination schedule for adults:
- Special population groups for vaccination who need special attention?
- What to do when a vaccination dose is missed?
- Individual vaccines – according to CDC updated vaccination schedule for adults

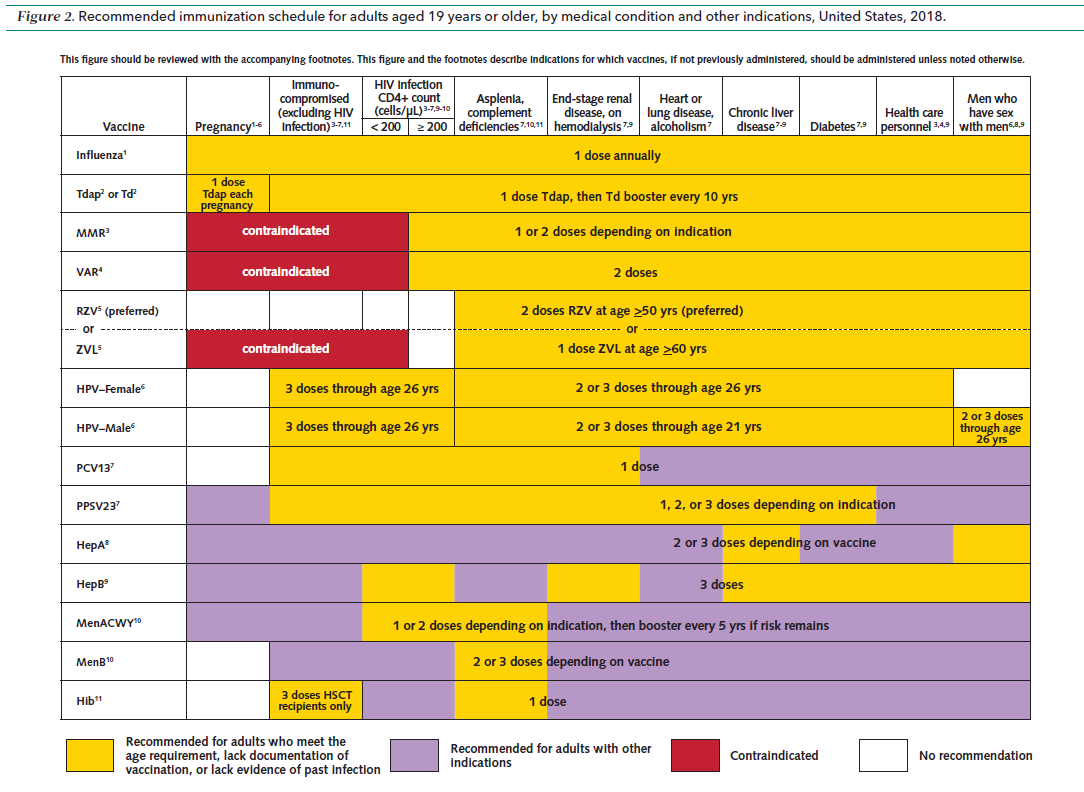
Updated vaccination schedule for adults – Individuals who need special attention prior to administering a vaccine?
Pregnant women.
Live vaccines like measles vaccine, mumps, and rubella vaccines are contraindicated during pregnancy.
pregnant women should receive tetanus, diphtheria and acellular pertussis vaccine (Tdap) during pregnancy and the influenza vaccine during or before pregnancy.
2. Asplenia (patients without spleen including those without a functional spleen)
Adults without spleen (post-splenectomy or those with functional asplenia including sickle cell disease and hemoglobinopathies) are at risk for infection by encapsulated organisms. All these patients should receive pneumococcal, meningococcal vaccine and vaccine for Hemophilus influenza. Furthermore, asplenic patients should seek urgent medical consultation when febrile.
3. Patients who are immunocompromised.
Definition of highly immunosuppressed individuals include:
- HIV (Human immunodeficiency virus) infected patients with a CD4 cell count of less than 200 cells/ul.
- Patients on steroid therapy, equivalent to 20 mg of prednisolone or more for 14 days or more.
- Patients with primary immunodeficiency disorders like Severe Combined immunodeficiency syndrome (SCID)
- Patients on chemotherapy for certain cancers.

Adult individuals who are immunosuppressed should generally avoid all live vaccines. Vaccination should be deferred for at least one month after discontinuation of immunosuppressive steroid therapy.
Contraindications and precautions applicable to all types of vaccines:

A severe reaction like anaphylaxis after a previous dose of a vaccine is a contraindication to that specific type of vaccine. Furthermore, vaccination should be delayed during a moderate to severe acute illness with or without a febrile state.
What to do when a vaccination dose is missed?
Vaccine efficacy is not altered with the vaccine combination and if the interval between doses is increased. Henceforth, it is not necessary to restart the vaccine series or add doses to the series because of an extended interval between doses.
Combination vaccines may be used when any component of the combination is indicated and when the other components of the combination are not contraindicated
CDC Updated Vaccination schedule for Adults
Influenza vaccination:
Vaccination for influenza virus is recommended for all individuals above 19 years of age
General information about influenza vaccination:
- Administer 1 dose of age-appropriate inactivated influenza vaccine (IIV) or recombinant influenza vaccine (RIV) annually
- Live attenuated influenza vaccine (LAIV) is not recommended for the 2017–2018 influenza season
Special populations
- Pregnant females and adults with hives-only egg allergy should be given age-appropriate IIV (inactivated influenza vaccine) or RIV (recombinant influenza vaccine)
- Adults with moderate to severe egg allergy other than hives (angioedema and respiratory distress) should be given IIV and RIV vaccine in a supervised medical setting by a health care provider who can manage severe allergic reactions.
Important precautions to take prior to administering IIV vaccine :
- History of Guillain-Barré syndrome within 6 weeks after previous influenza vaccination is a relative contra-indication to IIV vaccine and benefit/ hazards of the vaccine should be taken into account.

- Patients with egg allergy other than hives, e.g., angioedema, respiratory distress, lightheadedness, or recurrent emesis; or allergy requiring epinephrine or another emergency medical intervention should be administered the vaccine in a supervised setting where anaphylaxis can be managed.
Important precautions to take prior to administering the RIV vaccine include a history of Guillain-Barré syndrome within 6 weeks after a previous influenza vaccination.
Tdap and Td: tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine
Administer 1 dose of Tdap, followed by a dose of tetanus and diphtheria toxoids (Td) booster every 10 years
to adults who previously did not receive a dose of Tdap vaccine as an adult or child (routinely recommended at age 11–12 years)

Special populations
- Pregnant women: Administer 1 dose of Tdap during each pregnancy, preferably in the early part of gestational weeks 27–36.
Contraindications to Tdap:
Encephalopathy, e.g., coma, decreased level of consciousness, or prolonged seizures, not attributable to another identifiable cause within 7 days of administration of a previous dose of a vaccine containing tetanus or diphtheria toxoid or acellular pertussis is a contraindication to a pertussis-containing vaccine.
Precautions to take prior to administering Tdap vaccine include:
- Guillain-Barré syndrome within 6 weeks after a previous dose of tetanus toxoid-containing vaccine
- Vaccination should be deferred until at least 10 years have elapsed since the last tetanus toxoid-containing vaccine in patients with a history of Arthus-type hypersensitivity reactions after a previous dose of tetanus or diphtheria toxoid-containing vaccine.
- For the pertussis-containing vaccine, progressive or unstable neurologic disorder, uncontrolled seizures, or progressive encephalopathy (until a treatment regimen has been established and the condition has stabilized)

a seizure is a contraindication to Tdap vaccine
MMR (Measles, mumps, and rubella vaccination)
Administer 1 dose of measles, mumps, and rubella vaccine (MMR) to adults with no evidence of immunity to measles, mumps, or rubella
Evidence of immunity is:
Documentation of a health care provider-diagnosed disease without laboratory confirmation is not considered evidence of immunity
Special populations
- Pregnant women and nonpregnant women of childbearing age with no evidence of immunity to rubella:
Administer 1 dose of MMR (if pregnant, administer MMR after pregnancy and before discharge from health care facility)
- HIV infection and CD4 cell count ≥200 cells/μL for at least 6 months and no evidence of immunity to measles, mumps, or rubella:
Administer 2 doses of MMR at least 28 days apart
- Students in postsecondary educational institutions, international travelers, and household contacts of immunocompromised persons:
Administer 2 doses of MMR at least 28 days apart (or 1 dose of MMR if previously administered 1 dose of MMR)
- Health care personnel born in 1957 or later with no evidence of immunity:
Administer 2 doses of MMR at least 28 days apart for measles or mumps, or 1 dose of MMR for rubella (if born before 1957, consider MMR vaccination)
- Adults who previously received ≤2 doses of mumps-containing vaccine and are identified by the public health authority to be at increased risk for mumps in an outbreak:
Administer 1 dose of MMR
Contraindications to MMR
MMR should not be given to the following group of patients.
- Severe immunodeficiency, e.g., hematologic and solid tumors,
- Chemotherapy,
- Congenital immunodeficiency or long-term immunosuppressive therapy,
- Human immunodeficiency virus (HIV) infection.
- Pregnancy
Precautions to take prior to administering MMR vaccine:
- Recent (within 11 months) receipt of antibody-containing blood product (specific interval depends on product) (Vaccine should be deferred for the appropriate interval if replacement immune globulin products are being administered)
- History of thrombocytopenia or thrombocytopenic purpura
- Need for tuberculin skin testing (Measles vaccination may temporarily suppress tuberculin reactivity.
- The measles-containing vaccine may be administered on the same day as tuberculin skin testing, or should be postponed for at least 4 weeks after vaccination)
VAR (Varicella vaccine):
Administer to adults without evidence of immunity to varicella
2 doses of varicella vaccine (VAR) 4–8 weeks apart
Evidence of immunity to varicella is:
- U.S.-born before 1980 (except for pregnant women and health care personnel)
- Documentation of receipt of 2 doses of varicella or varicella-containing vaccine at least 4 weeks apart
- Diagnosis or verification of a history of varicella or herpes zoster by a health care provider
- Laboratory evidence of immunity or disease
Special populations to take caution
Administer 2 doses of VAR 4–8 weeks apart if previously received no varicella-containing vaccine (if previously received 1 dose of varicella-containing vaccine, administer 1 dose of VAR at least 4 weeks after the first dose) to:
- Pregnant women without evidence of immunity: Administer the first of the 2 doses or the second dose after pregnancy and before discharge from the healthcare facility
- Health care personnel without evidence of immunity
- Adults with HIV infection and CD4 cell count ≥200 cells/μL: May administer, based on the individual clinical decision, 2 doses of VAR 3 months apart
VAR is contraindicated for pregnant women and adults with severe immunodeficiency
Contraindications to varicella vaccination:
- Severe immunodeficiency, e.g., hematologic and solid tumors, chemotherapy, congenital immunodeficiency or long-term immunosuppressive therapy, HIV infection with severely immunocompromised state
- Pregnancy
Precautions to take:
- Recent (within 11 months) receipt of antibody-containing blood product (specific interval depends on the product)
- Receipt of specific antiviral drugs (acyclovir, famciclovir, or valacyclovir) 24 hours before vaccination (avoid the use of these antiviral drugs for 14 days after vaccination)
ZVL (zoster vaccine live):
- Administer 2 doses of recombinant zoster vaccine (RZV) 2–6 months apart to adults aged 50 years or older regardless of past episode of herpes zoster or receipt of zoster vaccine live (ZVL)
- Administer 2 doses of RZV 2–6 months apart to adults who previously received ZVL at least 2 months after ZVL
- For adults aged 60 years or older, administer either RZV or ZVL (RZV is preferred)
Special populations
- ZVL is contraindicated for pregnant women and adults with severe immunodeficiency
Contraindications:
- Severe immunodeficiency, e.g., hematologic and solid tumors, chemotherapy, congenital immunodeficiency or long-term immunosuppressive therapy3, HIV infection with severe immunocompromise
- Pregnancy
Precautions to take:
Receipt of specific antiviral drugs (acyclovir, famciclovir, or valacyclovir) 24 hours before vaccination (avoid the use of these antiviral drugs for 14 days after vaccination)
Human Papillomavirus (HPV) vaccine:
- Administer human papillomavirus (HPV) vaccine to females through age 26 years and males through age 21 years (males age 22 through 26 years may be vaccinated based on an individual clinical decision)
- Recently HPV vaccine (Gardasil 9) use has been expanded beyond 26 years of age and the FDA has recommended its use till 45 years of age
- The number of doses of HPV vaccine to be administered depends on age at initial HPV vaccination

No previous dose of HPV vaccine:
Administer 3-dose series at 0, 1–2, and 6 months (minimum intervals: 4 weeks between doses 1 and 2, 12 weeks between doses 2 and 3, and 5 months between doses 1 and 3; repeat doses if given too soon)
2. Aged 9–14 years at HPV vaccine series initiation and received 1 dose or 2 doses less than 5 months apart:
Administer 1 dose
3. Aged 9–14 years at HPV vaccine series initiation and received 2 doses at least 5 months apart:
No additional dose is needed Special populations
4. Adults with immunocompromising conditions (including HIV infection) through age 26 years:
Administer 3-dose series at 0, 1–2, and 6 months
5. Men who have sex with men through age 26 years:
Administer 2- or 3-dose series depending on age at initial vaccination (see above); if no history of HPV vaccine, administer 3-dose series at 0, 1–2, and 6 months
6. Pregnant women through age 26 years:
HPV vaccination is not recommended during pregnancy, but there is no evidence that the vaccine is harmful and no intervention needed for women who inadvertently receive HPV vaccine while pregnant; delay remaining doses until after pregnancy; pregnancy testing is not needed before vaccination
Pneumococcal vaccine (PCV 13):
Administer to immunocompetent adults aged 65 years or older
1 dose of 13-valent pneumococcal conjugate vaccine (PCV13), if not previously administered, followed by 1 dose of 23-valent pneumococcal polysaccharide vaccine (PPSV23) at least 1 year after PCV13
if PPSV23 was previously administered but not PCV13, administer PCV13 at least 1 year after PPSV23
- When both PCV13 and PPSV23 are indicated, administer PCV13 first (PCV13 and PPSV23 should not be administered during the same visit)
Special populations
- Administer to adults aged 19 through 64 years with the following chronic conditions:
1 dose of PPSV23
- Chronic heart disease (excluding hypertension)
- Chronic lung disease
- Chronic liver disease
- Alcoholism
- Diabetes mellitus
- Cigarette smoking

Administer to adults aged 19 years or older with the following indications
1 dose of PCV13 followed by 1 dose of PPSV23 at least 8 weeks after PCV13, and a second dose of PPSV23 at least 5 years after the first dose of PPSV23
(if the most recent dose of PPSV23 was administered before age 65 years, at age 65 years or older, administer another dose of PPSV23 at least 5 years after the last dose of PPSV23):
- Immunodeficiency disorders (including B- and T-lymphocyte deficiency, complement deficiencies, and phagocytic disorders
- HIV infection
- Anatomical or functional asplenia (including sickle cell disease and other hemoglobinopathies)
- Chronic renal failure and nephrotic syndrome
Administer to adults aged 19 years or older with the following indications:
1 dose of PCV13 followed by 1 dose of PPSV23 at least 8 weeks after PCV13 (if the dose of PPSV23 was administered before age 65 years, at age 65 years or older, administer another dose of PPSV23 at least 5 years after the last dose of PPSV23)
- Cerebrospinal fluid leak
- Cochlear implant
Hepatitis A vaccination
- Administer to adults who have a specific risk (see below), or lack a risk factor but want protection,
2-dose series of single-antigen hepatitis A vaccine (HepA; Havrix at 0 and 6–12 months or Vaqta at 0 and 6–18 months; minimum interval: 6 months)
or a
3-dose series of combined hepatitis A and hepatitis B vaccine (HepA-HepB) at 0, 1, and 6 months; minimum intervals: 4 weeks between first and second doses, 5 months between second and third doses

Special populations
- Administer HepA to adults with the following indications:
- Travel to or work in countries with high or intermediate hepatitis A endemicity
- Men who have sex with men
- Injection or non-injection drug use
- Work with hepatitis A virus in a research laboratory or with nonhuman primates infected with hepatitis A virus
- Clotting factor disorders
- Chronic liver disease
- Close, personal contact with an international adoptee (e.g., household or regular babysitting) during the first 60 days after arrival in the United States from a country with high or intermediate endemicity (administer the first dose as soon as the adoption is planned)
- Healthy adults through age 40 years who have recently been exposed to hepatitis A virus; adults older than age 40 years may receive HepA or HepA-HepB if hepatitis A immunoglobulin cannot be obtained
Hepatitis B vaccination
- Administer to adults who have a specific risk, or lack a risk factor but want protection
3-dose series of single-antigen hepatitis B vaccine (HepB) or combined hepatitis A and hepatitis B vaccine (HepA-HepB) at 0, 1, and 6 months (minimum intervals:
4 weeks between doses 1 and 2 for HepB and HepA-HepB;
between doses 2 and 3, 8 weeks for HepB and 5 months for HepA-HepB)
Special populations
Administer HepB or HepA-HepB to adults with the following indications:
- Chronic liver disease (e.g., hepatitis C infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, alanine aminotransferase [ALT] or aspartate aminotransferase [AST] level greater than twice the upper limit of normal)
- HIV infection
- Percutaneous or mucosal risk of exposure to blood (e.g., household contacts of hepatitis B surface antigen [HBsAg]-positive persons;
- Adults younger than age 60 years with diabetes mellitus or aged 60 years or older with diabetes mellitus based on an individual clinical decision;
- Adults in predialysis care or receiving hemodialysis or peritoneal dialysis;
- Recent or current injection drug users;
- Health care and public safety workers at risk for exposure to blood or blood-contaminated body fluids)
- Sexual exposure risk (e.g., sex partners of HBsAg-positive persons; sexually active persons not in a mutually monogamous relationship; persons seeking evaluation or treatment for a sexually transmitted infection; and men who have sex with men [MSM])
- Receive care in settings where a high proportion of adults have risks for hepatitis B infection (e.g., facilities providing sexually transmitted disease treatment, drug abuse treatment and prevention services, hemodialysis and end-stage renal disease programs, institutions for developmentally disabled persons, health care settings targeting services to injection drug users or MSM, HIV testing and treatment facilities, and correctional facilities)
- Travel to countries with high or intermediate hepatitis B endemicity
Meningococcal vaccination
Serogroups A, C, W, and Y meningococcal vaccine (MenACWY)
Administer to adults with the following indications:
2 doses of MenACWY at least 8 weeks apart and revaccinate with 1 dose of MenACWY every 5 years, if the risk remains
- Anatomical or functional asplenia (including sickle cell disease and other hemoglobinopathies)
- HIV infection
- Persistent complement component deficiency
- Eculizumab use
Administer 1 dose of MenACWY and revaccinate with 1 dose of MenACWY every 5 years, if the risk remains, to adults with the following indications:
- Travel to or live in countries where meningococcal disease is hyperendemic or epidemic, including countries in the African meningitis belt or during the Hajj
- At risk from a meningococcal disease outbreak attributed to serogroup A, C, W, or Y
- Microbiologists routinely exposed to Neisseria meningitidis
- Military recruits
- First-year college students who live in residential housing (if they did not receive MenACWY at age 16 years or older)
Haemophilus influenza type b vaccination
Administer Haemophilus influenza type b vaccine (Hib) to adults with the following indications:
- Anatomical or functional asplenia (including sickle cell disease) or undergoing elective splenectomy: Administer 1 dose if not previously vaccinated (preferably at least 14 days before elective splenectomy)
- Hematopoietic stem cell transplant (HSCT): Administer 3-dose series with doses 4 weeks apart starting 6 to 12 months after successful transplant regardless of Hib vaccination history
Read more on
1. Vaccination in children
2. HPV vaccination – Gardasil 9 beyond ages
3. Chronic lung diseases – All that wheezes is not ASTHMA!