ARNI – AR stands for Angiotensin receptor blocker and NI stands for Neprilysin Inhibitor.
In patients with heart failure, the levels of atrial and brain natriuretic peptides rise when the cardiac muscles are stretched because of fluid overload. An increase in ANP and BNP levels in the blood results in a diuretic effect.
Thus, these hormones are very important in patients with heart failure. However, an enzyme called Neprilysin causes the degradation of ANP and BNP resulting in worsening of the symptoms.
The new drug sacubitril which inhibits the enzyme neprilysin indirectly cause an elevation in the levels of ANP and BNP. In combination with valsartan, which reduces cardiac preload i.e. the volume of blood coming into the heart as well as prevents cardiac remodeling, it is shown to prevent sudden cardiac deaths and improve mortality.
ARNI vs Enalapril in heart failure – The Paradigm-HF trial:
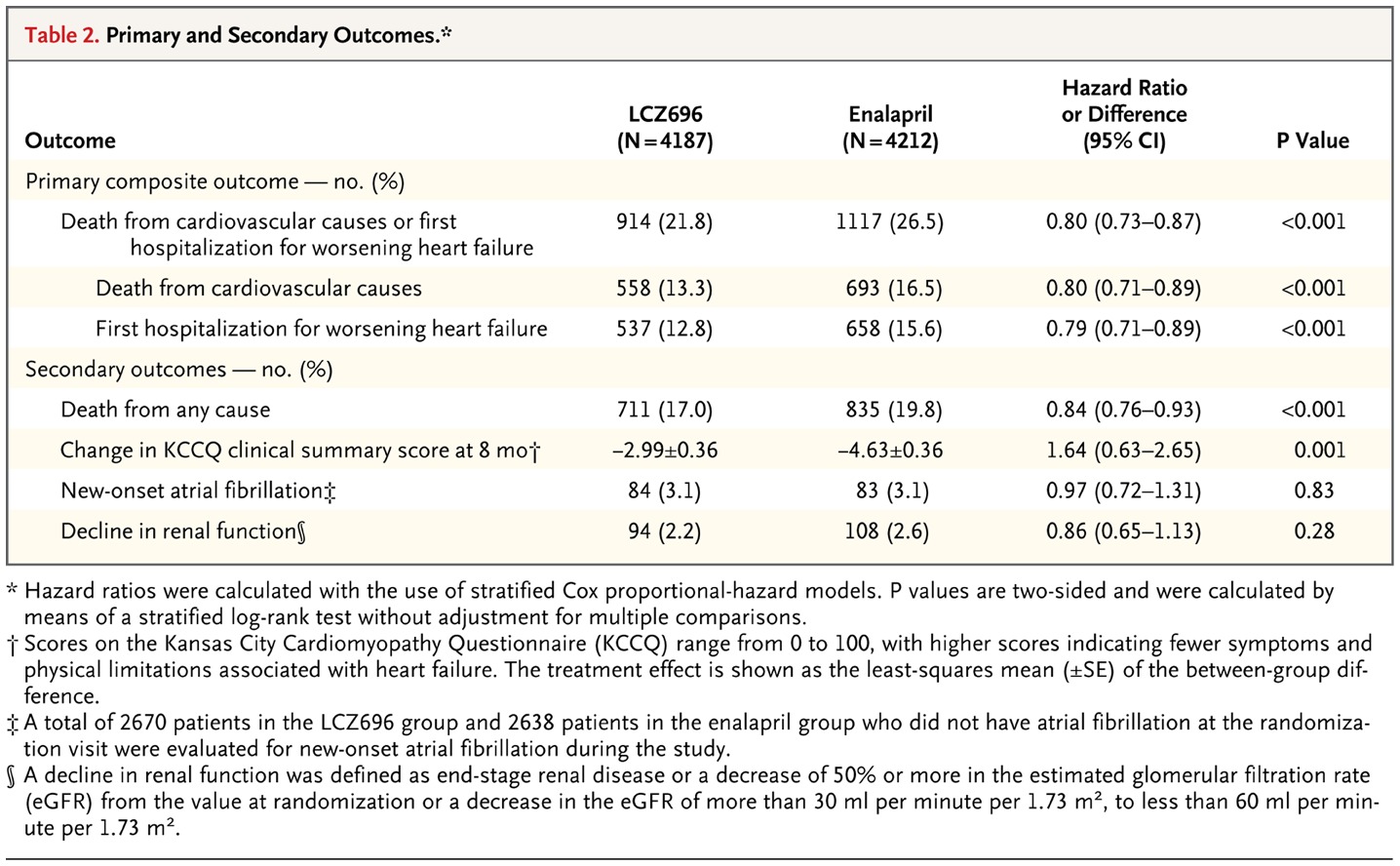
The recent study “Paradigm-HF” in which enalapril was compared with sacubitril/valsartan, comprised of more than 8000 patients, in a randomized manner.
The study was conducted in patients with heart failure and reduced ejection fraction. The trial was stopped early because the boundary for an enormous benefit with ARNI was crossed.
Patients in the ARNI (Sacubitril/valsartan) had a 20% relative risk reduction in the primary outcomes of deaths from a cardiovascular cause or hospitalization due to heart failure.
The 20 % risk reduction was independent of protection with implantable cardioverter defibrillator.
Furthermore, all patients were on guidelines guided therapy for heart failure. Most patients were on beta blockers and almost half of them were using mineralocorticoid receptor blockers.
ARNI also reduced hospitalization due to heart failure by 21% compared to enalapril. Patients in the sacubitril/valsartan group had a greater chance of non-serious angioedema and hypotension and a lower risk of cough, renal impairment, and hyperkalemia.
ARNI might alleviate the need for an ICD in patients with heart failure
Almost half of the patients with heart failure and an ejection fraction of < 35% die of sudden cardiac deaths.
Till date, ICD, although recommended in patients with an ejection fraction of less than 35%, has not been shown to reduce mortality in patients with heart failure.
The use of sacubitril/valsartan in patients with heart failure might alleviate the need for an implantable cardioverter-defibrillator.
Sacubitril/valsartan marketed by Novartis in Pakistan
Novartis has marketed the sacubitril/valsartan complex by the name Uperio in two strengths 100 mg and 200 mg tablets each.
- The 100 mg tablets contain 49 mg sacubitril and 51 mg valsartan
- The 200 mg tablets contain 97 mg sacubitril and 103 mg valsartan.
- The recommended dose is 100 mg twice daily which may be increased to 200 mg twice daily after two to four weeks.
- 28 tablets-pack costs Rs.1900/-
Uperio/ Entresto is a complex and not just a combination of sacubitril and valsartan:
Pharmevo pharmaceuticals have also launched the drug. Novartis Pharma has raised an objection and a letter to DRAP (Drug regulatory authority of Pakistan) has been written regarding Uperio in comparison to the local brands.
The brand leader emphasizes that Uperio is not a combination of sacubitril and valsartan, it is rather a complex of the two drugs. Contrary to Uperio, the local brands have made a mixture or a combination of the two drugs, the efficacy of which is objectionable.