Vaccination plays a pivotal role in the prevention of deadly infectious diseases. Parents, institutions, and governments should follow the WHO updated vaccination schedule for children and adults.
What is a vaccine?
A vaccine is an antigenic substance that induces immunity when given to a person as an injection or in oral form.
The antigenic substance can be derived from an inactive form of the causative agent or a synthetic material. A vaccine stimulates the body’s own immune system to protect the person against subsequent infection or disease.
What are the different types of vaccines?
Vaccines can be divided into four different types:
Live attenuated vaccines
- BCG vaccine
- Oral Polio vaccine
- Measles vaccine
- Rotavirus vaccine
- Yellow fever vaccine
Inactivated or killed vaccine
- Inactivated polio vaccine
- Whole cell Pertussis vaccine
Subunit (purified antigen)
- Acellular pertussis
- Haemophilus influenza type b (Hib)
- Pneumococcal vaccine
- Hepatitis B vaccine
Toxoid (inactivated toxins)
- Tetanus toxoid
- Diphtheria toxoid
Which diseases are vaccine preventable?
As the name suggests, all the infectious diseases for which vaccines are available like:
- Polio,
- measles,
- mumps,
- rubella,
- diphtheria,
- whooping cough,
- Hepatitis A,
- Hepatitis B,
- Tetanus,
- typhoid fever,
- cholera,
- rotavirus gastroenteritis,
- pneumonia due to pneumococcal infection
- Haemophilus infections and
- even cervical cancer are vaccine-preventable.
Why is vaccination important?
Vaccination is important to prevent some life-threatening and many disabling diseases.
Like smallpox which has been eradicated, polio is very close to eradication as well. The last case of smallpox was identified in Somalia in 1977.
Pakistan and Afghanistan are the only two countries where polio cases are still being identified.
Similarly, pneumonia in elderly patients and those with chronic lung diseases can be life-threatening. Prior vaccination can prevent pneumonia from pneumococcal infections in the elderly.
Which group of patients should not be vaccinated?
Patients with advanced HIV infection, those with very low immunity and those with hypogammaglobulinemia should not be given live attenuated vaccines.
This poses the risk of infection and adverse effects from that very vaccine.
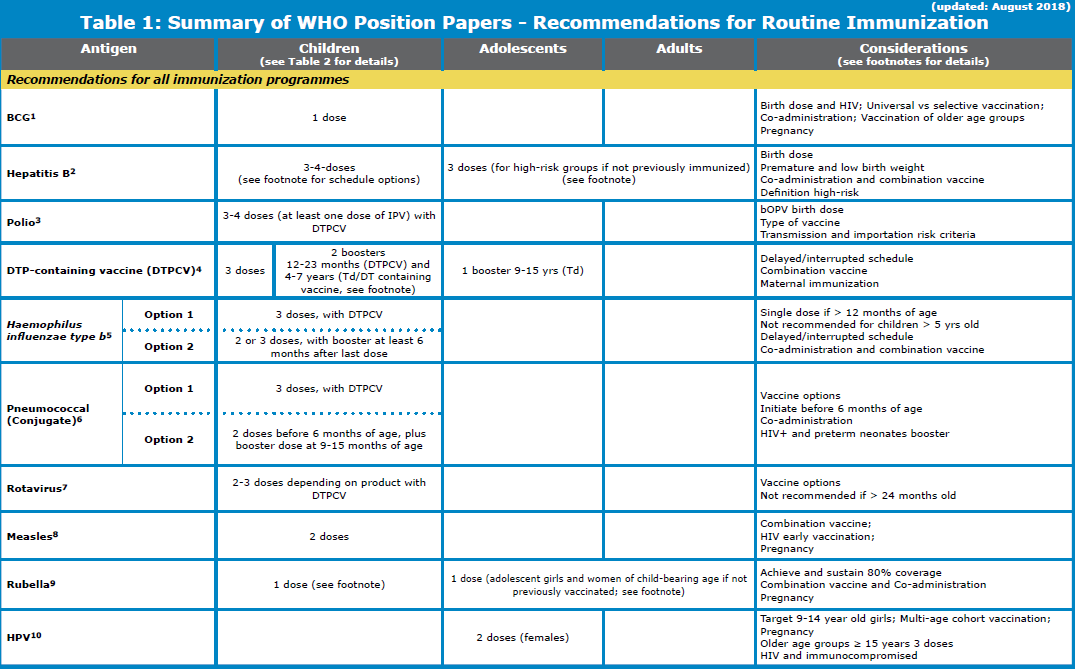
WHO 2018 updated vaccination schedule for children
BCG (Bacillus Calmette–Guérin vaccine):
BCG is a vaccine used for the prevention of tuberculosis.

Tuberculosis is caused by Mycobacterium tuberculosis. It can affect any organ commonly the lungs, lymph nodes, gastrointestinal tract, joints, and nervous system.
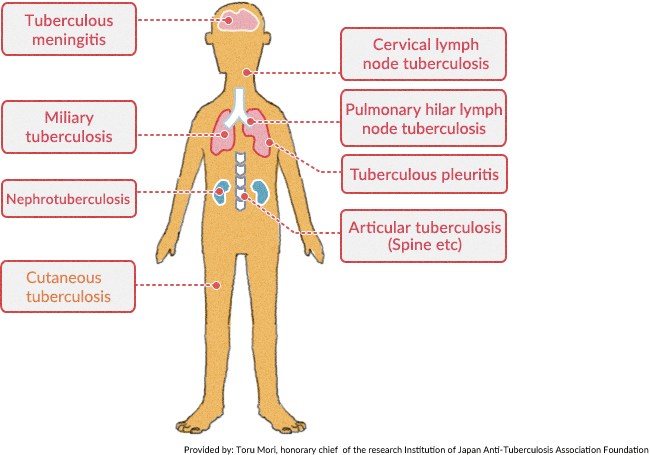
Patients with pulmonary TB usually have a chronic fever with night sweats, productive cough, and weight loss. With the HIV epidemic in the developed world, a resurgence in tuberculosis has been noted.
Thus BCG vaccination is not only important in the developing world but also in the western world.
The WHO updated vaccination schedule for children recommends BCG, oral polio and Hepatitis B vaccine to be administered soon after birth.
BCG is a live vaccine
When to administer: at birth
Dose of BCG: 0.05ml
Route of administration: Intradermal in right deltoid.
Who Should be vaccinated?
BCG vaccine should be given routinely to newborns in countries with a high incidence of tuberculosis and leprosy. Ideally, this should be given at birth along with hepatitis B vaccine.
BCG vaccination in HIV infected patients:
Since BCG is a live vaccine, severely immunocompromised patients like children with advanced HIV infection and AIDS should not receive BCG vaccination.

All HIV-infected individuals who have a CD4% of more than 25% (age less than 5 years) or a CD4 count of more than 200/ul (age more than 5 years) should be vaccinated with BCG.
Children born to women of unknown HIV status should be vaccinated as the benefits of BCG vaccination outweigh the risks.
Neonates, born to HIV infected mothers, without any clinical evidence of HIV infection should also receive BCG vaccination, regardless of whether the mother is receiving antiretroviral therapy.
BCG vaccination in preterm infants:
Infants with low birth weight (<2500 gm or 2.5 kilograms) and moderate-to-late preterm infants (gestational age > 31 weeks) who are healthy and clinically stable can receive BCG vaccination at birth, or at the latest, upon discharge.
Who should not receive BCG vaccination?
BCG vaccination is not recommended during pregnancy.
For HIV confirmed neonate, BCG should be delayed until antiretroviral therapy has been started and the infant is immunologically stable (CD4 counts >25%).
When is BCG vaccination optional?
Countries with low TB incidence or leprosy burden may choose to selectively vaccinate neonates in high-risk groups.
Hepatitis B vaccination:
Hepatitis B is caused by the hepatitis B virus. The hepatitis B virus is commonly transmitted through body fluids such as blood, semen, and vaginal secretion.
Its clinical presentation “ anorexia, nausea and vomiting, low-grade fever, myalgia, arthralgia, fatigability, right upper quadrant, and epigastric pain. Hepatitis b can complicate into Chronic liver disease and hepatocellular carcinoma.
Hepatitis B vaccination is recommended worldwide for all children.
How many doses of hepatitis B vaccine are required?
At least 3 doses of hepatitis B vaccine should be administered to the child and should be a standard for all national immunization programs.
Since maternal to fetal transmission is the most important source of chronic hepatitis B infection, all infants should receive Hepatitis B vaccine, ideally within 24 hours of birth. These include premature and low birth weight infants.
The Hepatitis B vaccine should be repeated at four weeks interval 2 to 3 times. An additional 4th dose if given does not pose any risk to the child.
The interval between doses should be at least 4 weeks.
- Premature and low birth weight infants (less than 2 kilograms) can be given the hepatitis B vaccine at birth. These infants should receive a total of four doses.
Hepatitis B Vaccine summary:
It consists of a purified inactive subunit of the virus and is not infectious
It is not contraindicated in immunosuppressed person and the pregnant woman.
It is 85% effective in preventing perinatal infections.
3 doses of hepatitis B vaccine are to be given via intramuscular route.
Doses: <19 years = 0.5 ml, >19 years = 1 ml
Polio vaccination schedule:
Polio is an infectious disease caused by the poliovirus. Most of the patient with polio have no symptoms.
However, the most common presentation of polio is “ fever, headache, sore throat and weakness or paralysis of the muscles of the legs, hands, and even the muscles of swallowing and respiration.
Polio can result in permanent weakness and disability. It spreads mostly via contaminated water. Other sources include unhygienic food that contains the poliovirus.
OPV (oral polio vaccine) plus IPV (injectable polio vaccine):
- WHO updated vaccination schedule recommends the addition of at least one dose of injectable polio vaccine for all countries using OPV in the national immunization program.
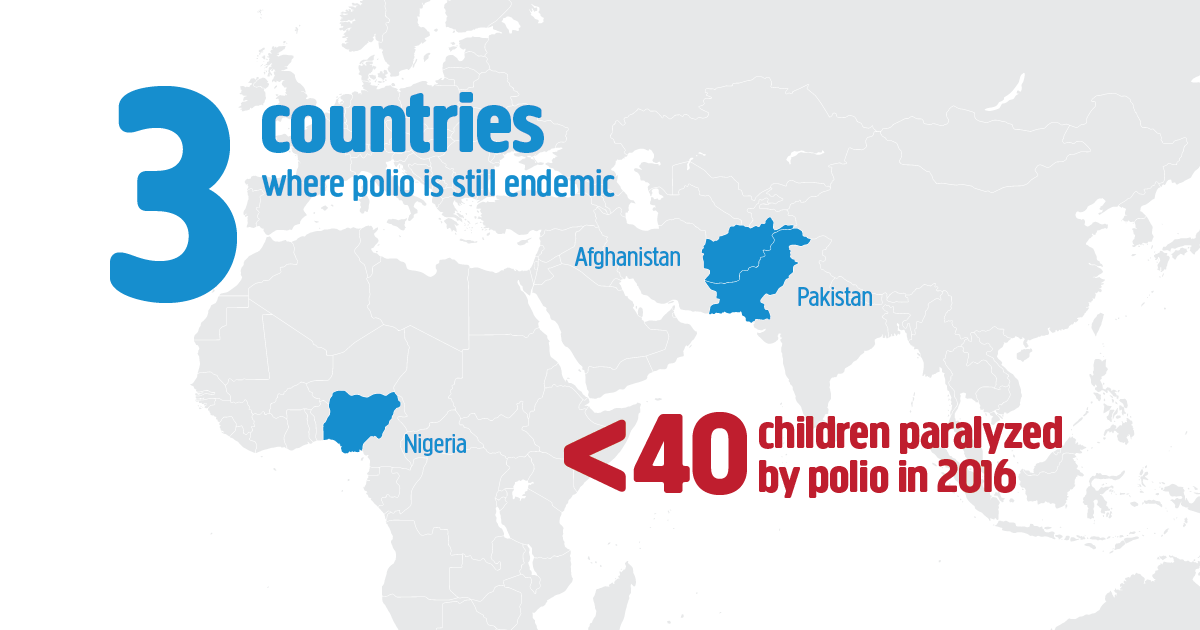
- In polio-endemic countries like Pakistan and Afghanistan and in countries at high risk for importation and subsequent spread of poliovirus, WHO recommends a bOPV (bivalent Oral polio vaccine) birth dose (zero dose) followed by a primary series of 3 bOPV doses and at least 1 IPV dose.
- The zero dose of bOPV should be administered at birth, or as soon as possible after birth, to maximize seroconversion rates following subsequent doses and to induce mucosal protection.
- The primary series consisting of 3 bOPV doses plus 1 IPV dose can be initiated from the age of 6 weeks with a minimum interval of 4 weeks between the bOPV doses.
- If 1 dose of IPV is used, it should be given at 14 weeks of age or later and can be co-administered with a bOPV dose.
- The primary series can be administered according to the regular schedules of national immunization programs, e.g. at 6, 10, and 14 weeks (bOPV, bOPV, bOPV+IPV), or at 2, 4, and 6 months.
- Both OPV and IPV may be co-administered with other infant vaccines.
IPV-only schedule
- An IPV-only schedule may be considered in countries with sustained high vaccination coverage and very low risk of both WPV importation and transmission.
- A primary series of 3 doses of IPV should be administered beginning at 2 months of age. If the primary series begins earlier (e.g. with a 6, 10 and 14-week schedule) then a booster dose should be given after an interval of ≥6 months (for a 4-dose schedule).
DTP (Diphtheria, tetanus, and pertussis) vaccine schedule
Diphtheria is an infectious disease caused by Corynebacterium diphtheria. It can involve the respiratory system, cardiovascular system, nervous system, and kidneys.
The clinical presentation of diphtheria is ‘’ a sore throat, malaise, cervical lymphadenopathy, low-grade fever and white exudate on tonsils that are adherent to tonsils forming pseudomembrane which bleed with scraping.
The serious complication of diphtheria is involving heart causing myocarditis. This disease is also preventable with vaccination.
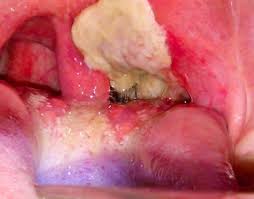
The need for early infant vaccination with DTP-containing vaccine (DTPCV) is principally to ensure rapid protection against pertussis because severe disease and death from pertussis are almost entirely limited to the first weeks and months of life.
A primary series of 3 doses of DTP-containing vaccine is recommended, with the first dose administered as early as 6 weeks of age.
Subsequent doses should be given with an interval of at least 4 weeks between doses. The third dose of the primary series should be completed by 6 months of age if possible.
- If either the start or the completion of the primary series has been delayed, the missing doses should be given at the earliest opportunity with an interval of at least 4 weeks between doses.
- 3 booster doses of diphtheria toxoid-containing vaccine should be provided during childhood and adolescence.
- The diphtheria booster doses should be given in combination with tetanus toxoid using the same schedule, i.e at 12–23 months of age, 4–7 years of age, and 9–15 years of age, using age-appropriate vaccine formulations.
Ideally, there should be at least 4 years between booster doses.
Tetanus Vaccination – Tetanus toxoid
- To ensure lifelong protection against tetanus in all people should receive 6 doses (3 primary plus 3 booster doses) of tetanus toxoid-containing vaccine (TTCV) through routine childhood immunization schedules.

- The 3 TTCV booster doses should be given at 12–23 months of age; 4–7 years of age; and 9–15 years of age. Ideally, there should be at least 4 years between booster doses.
- National vaccination schedules can be adjusted within the age limits specified above to enable programs to tailor their schedules based on local epidemiology, the objectives of the immunization program, any particular programmatic issues and to better align tetanus vaccination with the immunological requirements of other vaccines (particularly for pertussis and diphtheria).
To provide and sustain both tetanus and diphtheria immunity throughout the life course and for both sexes, age-appropriate combinations of tetanus and diphtheria toxoids should be used.
For children, less than 7 years of age DTwP or DTaP combinations may be used. For children aged 4 years and older Td containing vaccine may be used and is preferred.

- From 7 years of age, only Td combinations should be used. Age-appropriate combinations containing pertussis vaccine with low-dose diphtheria antigen are also available.
- If tetanus vaccination is started during adolescence or adulthood, a total of only 5 appropriately spaced doses are required to obtain lifelong protection.
Pertussis vaccine:
Pertussis is caused by Bordetella pertussis which is a highly contagious acute respiratory tract illness. It is also called whooping cough.
The clinical presentation of this disease is a sudden attack of coughing, inspiratory whoop, and post-tussive vomiting. The symptoms may vary in the vaccinated individual.
Both aP-containing (acellular pertussis) and wP-containing (whole-cell pertussis) vaccines have excellent safety records.
- Available evidence indicates that licensed aP and wP vaccines have equivalent initial effectiveness in preventing disease in the first year of life, but that there is a more rapid waning of immunity, and possibly a reduced impact on transmission, with aP relative to wP vaccines.

- National programs currently administering wP vaccination should continue to use wP vaccines for primary vaccination series. Surveillance and modeling data suggest that the use of aP vaccines may result in a resurgence of pertussis after a number of years.
- National programs currently using aP vaccine may continue using this vaccine but should consider the need for additional booster doses and strategies to prevent early childhood mortality such as maternal immunization in case of a resurgence of pertussis.
Only aP-containing vaccines should be used for vaccination of persons aged ≥7 years.
- Pertussis-containing booster – A booster dose is recommended for children aged 1–6 years, preferably during the second year of life (≥6 months after last primary dose) unless otherwise indicated by local epidemiology; the contact could also be used to catch up on any missed doses of other vaccines.

- This schedule should provide protection for at least 6 years for countries using wP vaccine. For countries using aP vaccine, protection may decline appreciably before 6 years of age.
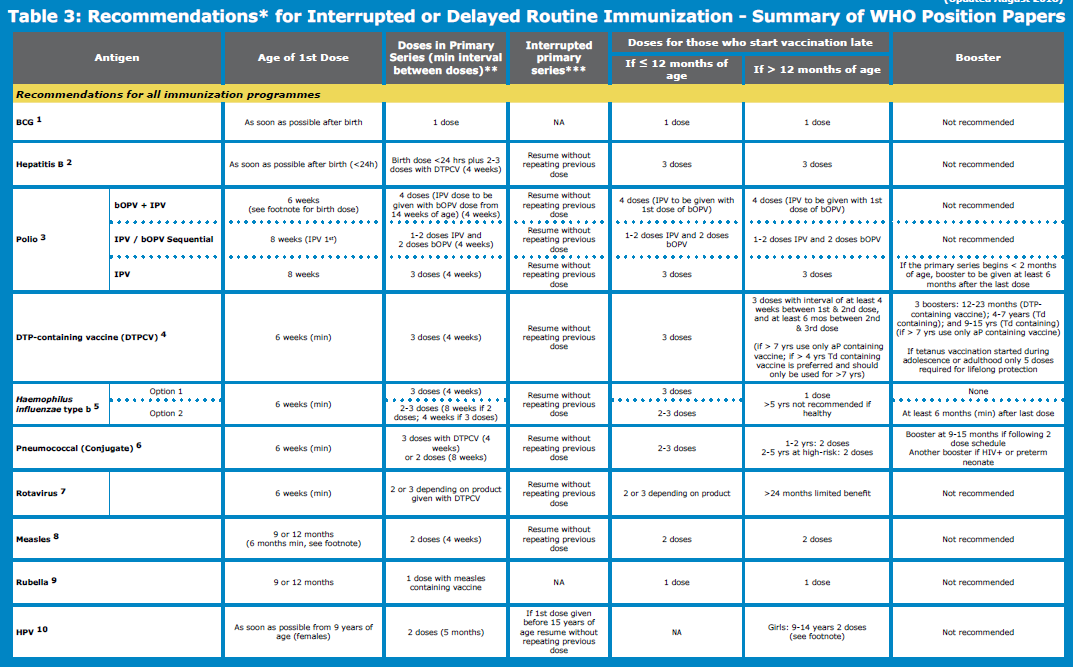
Delayed or interrupted DTP-containing series:
- For children whose vaccination series has been interrupted, the series should be resumed without repeating previous doses. Children aged 1 to < 7 years who have not previously been vaccinated should receive 3 doses of vaccine following a 0, 1, 6-month schedule.
- Two subsequent booster doses using Td or Tdap combination vaccines are needed with an interval of at least 1 year between doses.

Hemophilus influenza vaccination schedule
The use of Hib vaccines should be part of a comprehensive strategy to control pneumonia including exclusive The comprehensive strategy to control pneumonia apart from vaccination includes:
breastfeeding for six months,
handwashing with soap,
improved water supply and sanitation,
reduction of household air pollution, and
improved case management at community and health facility levels.
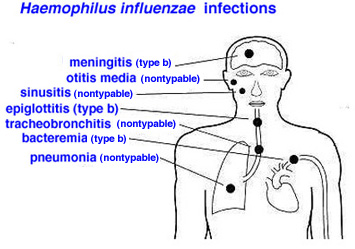
- WHO recommends that any one of the following Hib immunization schedules may be followed:
- 3 primary doses without a booster (3p);
- 2 primary doses plus a booster (2p+1); and
- 3 primary doses with a booster (3p+1).
- Because serious Hib disease occurs most commonly in children aged between 4 months and 18 months, immunization should start from 6 weeks of age, or as early as possible thereafter.
- In countries where the peak burden of severe Hib disease occurs in young infants, providing 3 doses of vaccine early in life may confer a greater benefit.
- In some settings (e.g. where the greatest disease morbidity and mortality occur later, or where rate reductions of the disease are not fully sustained after the routine use of Hib vaccine), it might be advantageous to give a booster dose by following either a 2p+1 or 3p+1 schedule.
- The interval between doses should be at least 4 weeks if 3 primary doses are given, and at least 8 weeks if 2 primary doses are given.
- Booster doses should be administered at least six months after completion of the primary series.
If the vaccination course has been interrupted, the schedule should be resumed without repeating the previous dose.
Children who start vaccination late, but are aged under 12 months, should complete the vaccination schedule (e.g. have 3 primary doses or 2 primary doses plus a booster).
- When a first dose is given to a child older than 12 months of age, only one dose is recommended.
- Hib vaccine is not required for healthy children after 5 years of age.
- The Hib conjugate vaccine is contraindicated in people with known allergies to any component of the vaccine. There are no other known contraindications or precautions.
Pneumococcal vaccination schedule
- Pneumococcal infection is caused by Streptococcus pneumoniae. Streptococcus pneumonia is the most common cause of community-acquired pneumonia.
- It can also cause meningitis, bacteremia, joint, and bone infection.
- Children over 2 years who are at high risk ( those with sickle cell disease, chronic renal failure, immunosuppression from organ transplantation, HIV infection or who undergoing splenectomy.
- Pneumococcal conjugate vaccines (PCVs) are considered safe in all target groups for vaccination, also in immunocompromised individuals.
- When injected at different sites, PCVs can be administered concurrently with any other vaccines in infant immunization programs.
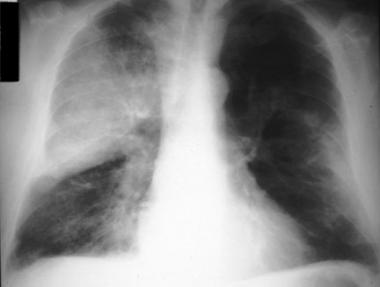
- For infants, 3 primary doses (the 3p+0 schedule) or, as an alternative, 2 primary doses plus a booster (the 2p+1 schedule).
- Higher antibody levels are induced by the third (booster) dose in a 2p+1 schedule compared to the third dose in a 3p+0 schedule. This may be important for the duration of protection or effectiveness against some serotypes.
- If the 3p+0 schedule is used, vaccination can be initiated as early as 6 weeks of age with an interval between doses of 4–8 weeks, depending on programmatic convenience.
- If the 2p+1 schedule is selected, the 2 primary doses should ideally be completed by six months of age, starting as early as 6 weeks of age with a minimum interval of 8 weeks or more between the two doses (for infants aged ≥7 months a minimum interval of 4 weeks between doses is possible). One booster dose should be given between 9–15 months of age.
- Previously unvaccinated or incompletely vaccinated children (including those who had a laboratory-confirmed invasive pneumococcal disease) should be vaccinated using the recommended age-appropriate regimens. Interrupted schedules should be resumed without repeating the previous dose.
- HIV-positive infants and pre-term neonates who have received their 3 primary vaccine doses before reaching 12 months of age may benefit from a booster dose in the second year of life.
Measles vaccination schedule:
- Measles is a highly contagious viral illness that occurs worldwide. The most typical clinical presentation of measles are “ fever, malaise, cough, coryza, conjunctivitis followed by an eruptive skin rash.
- Reaching all children with 2 doses of measles vaccine should be the standard for all national immunization programs.
- In addition to the first routine dose of MCV1, all countries should add a second routine dose of MCV2 to their national immunization schedules regardless of the level of MCV1 coverage.

- In countries with ongoing transmission in which the risk of measles mortality remains high, MCV1 should be given at age 9 months.
- MCV2 should be given between 15-18 months, as providing MCV2 in the 2nd year of life reduces the rate of accumulation of susceptible children and the risk of an outbreak. The minimum interval between MCV1 and MCV2 is 4 weeks.
- Every opportunity (e.g. when children come into contact with health services) should be taken to vaccinate all children that missed one or both MCV routine doses, particularly those under 15 years of age.
- In countries with low levels of measles transmission (i.e. those that are near elimination or verified as having eliminated endemic measles virus transmission) and therefore the risk of measles virus infection among infants is low, MCV1 may be administered at 12 months of age to take advantage of the higher seroconversion rates achieved at this age.
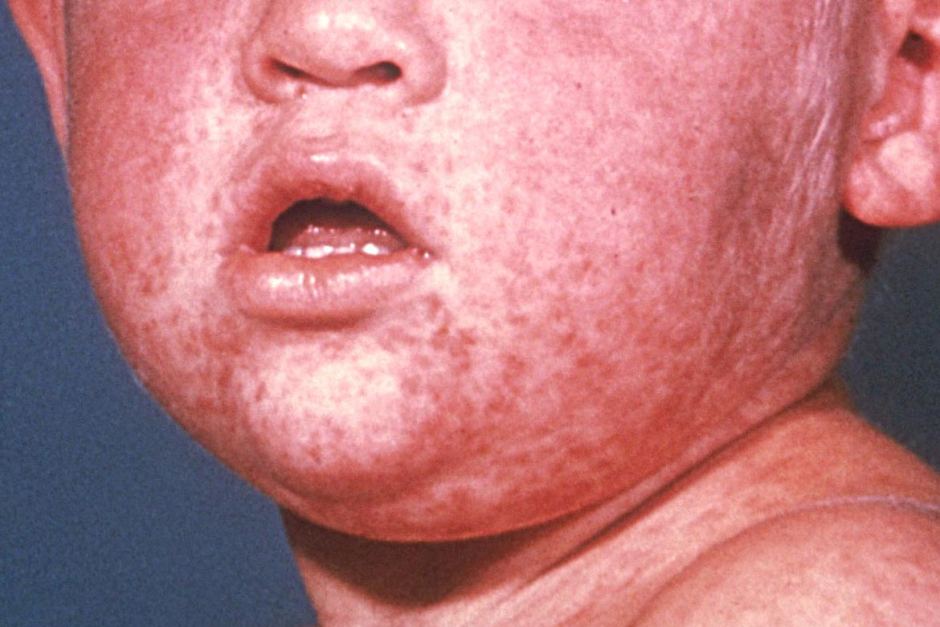
- In the following situations, a supplementary dose of MCV should be given to infants from 6 months of age:
- during a measles outbreak;
- during campaigns in settings where the risk of measles among infants < 9 months of age remains high (e.g. in endemic countries experiencing regular outbreaks);
- for internally displaced populations and refugees, and populations in conflict zones;
- for individual infants at high risk of contracting measles (e.g. contacts of known measles cases or in settings with an increased risk of exposure during outbreaks such as day-care facilities)
- Infants traveling to countries experiencing measles outbreaks;
- For infants known to be HIV-infected or exposed (i.e. born to an HIV-infected woman).
- MCV administered before 9 months of age should be considered a supplementary dose and recorded on the child’s vaccination record as “MCV0”. Children who receive MCV0 should also receive MCV1 and MCV2 at the recommended ages according to the national schedule.
Measles vaccination in HIV infected and immunocompromised individuals:
- Given the severe course of measles in patients with AIDS, measles vaccination should be routinely administered to potentially susceptible, asymptomatic HIV infected children and adults.
- Vaccination may even be considered for those with symptomatic HIV infection if they are not severely immunosuppressed according to conventional definitions. In areas where there is a high incidence of both HIV infection and measles, an initial dose of MCV may be offered as early as age 6 months (recorded as MCV0).
- The 2 routine doses of MCV (MCV1 and MCV2) should then be administered to these children according to the national immunization schedule.
- An additional dose of MCV should be administered to HIV-infected children receiving HAART following immune reconstitution. If CD4+ T lymphocyte counts are monitored, an additional dose of MCV should be administered when immune reconstitution has been achieved, e.g. when the CD4+ T lymphocyte count reaches 20–25%.
- Where CD4+ T lymphocyte monitoring is not available, children should receive an additional dose of MCV 6–12 months after initiation of HAART.
Supplementary doses in HIV infected individuals:
- A supplementary dose of MCV (recorded as MCV0) should be considered for infants known to be exposed (i.e. born to an HIV-infected woman) or soon after diagnosis of HIV infection in children older than 6 months who are not receiving HAART and for whom the risk of measles is high, with the aim of providing partial protection until they are revaccinated after immune reconstitution with HAART.
- Mild concurrent infections are not a contraindication to vaccination. As a precautionary measure, measles vaccine – alone or in combination with other vaccines – should be avoided during pregnancy.
- MCVs should not be given to individuals with a history of anaphylactic reactions or severe allergic reactions to any component of the vaccine (e.g. neomycin or gelatin) or those with any form of severe immunosuppression.
Rubella vaccination schedule
- Recommended for use in high performing immunization programs with the capacity to maintain coverage of over 80% and where mumps reduction is a public health priority.
- If implemented, a combination vaccine of measles, mumps, and rubella is recommended.
All countries that have not yet introduced rubella vaccine, and are providing 2 doses of measles vaccine using routine immunization, or SIAs, or both, should consider including rubella-containing vaccines (RCVs) in their immunization program.

- Because rubella is not as highly infectious as measles and because the effectiveness of 1 dose of an RCV is > 95% even at 9 months of age, only 1 dose of rubella vaccine is required to achieve rubella elimination if high coverage is achieved.
- However, when combined with measles vaccination, it may be easier to implement a second dose of RCV’s using the same combined MR vaccine or MMR vaccine for both doses.
- The first dose of RCV can be delivered at 9 or 12 months depending on the measles vaccination schedule.
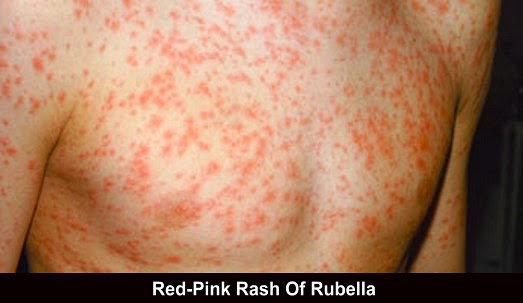
- Because of a theoretical, but never demonstrated, teratogenic risk rubella vaccination in pregnant women should be avoided in principle, and those planning a pregnancy are advised to avoid pregnancy for 1 month following vaccination.
- Administration of blood or blood products before or shortly after vaccination may interfere with vaccine efficacy. If using only rubella vaccines persons who received blood products should wait at least 3 months before vaccination and, if possible, blood products should be avoided for up to 2 weeks postvaccination.
- Vaccinated persons are not eligible to donate blood for 1 month after vaccination.
Rota virus vaccination schedule
Recommended to be included in all national immunization programmes.
- Early immunization is favored with the first dose of rotavirus vaccine to be administered from 6 weeks of age, however, in order to benefit those who may come late infants can receive doses without age restriction.
- Because of the typical age distribution of rotavirus gastroenteritis (RVGE), rotavirus vaccination of children >24 months of age is not recommended.
- Rotarix is administered orally in a 2-dose schedule at the time of DTPCV with an interval of at least 4 weeks between doses.
- RotaTeq vaccine is administered orally in a 3-dose schedule at the time of DTPCV contacts, with an interval of at least 4 weeks between doses.
- The rotavac vaccine is administered orally as a 3 dose schedule at the time of DTPCV, with an interval of at least 4 weeks between doses.
- Rotavirus vaccinations can be administered simultaneously with other vaccines in the infant immunization program.
Adverse effects and contraindications to rotavirus vaccination:
- Apart from a low risk of intussusception (about 1-2 per 100 000 infants vaccinated), the current rotavirus vaccines are considered safe and well tolerated.
- Severe allergic reaction (e.g. anaphylaxis) after a previous dose and severe immunodeficiency including severe combined immunodeficiency are contraindications for rotavirus vaccination.
- Precautions are necessary if there is a history of intussusception or intestinal malformations, chronic gastrointestinal disease, and severe acute illness. Vaccination should be postponed in case of ongoing acute gastroenteritis or fever with moderate to severe illness.
- The use of rotavirus vaccines should be part of a comprehensive strategy to control diarrhoeal diseases with the scaling up of both preventions (exclusive breastfeeding for 6 months, vitamin A supplementation, safe drinking water, hygiene/handwashing with soap, and sanitation) and treatment (low-osmolarity ORS, zinc and continued feeding).
Typhoid fever vaccination schedule:
Typhoid vaccination programs should be implemented in the context of other efforts to control the disease, including health education, water quality, and sanitation improvements, and training of health professionals in diagnosis and treatment.

- Among the available typhoid vaccines, TCV is preferred at all ages in view of its improved immunological properties, use in younger children and expected the duration of protection.
- Countries may consider the routine use of ViPS vaccine in individuals 2 years and older, and Ty21a vaccine for individuals more than 6 years of age.
- TCV – for infants and children from 6 months of age and in adults up to 45 years. Administration of TCV at the same time as other vaccine visits at 9 months of age or in the second year of life is encouraged.
- ViPS – single dose from 2 years of age. Ty21a – 3-doses to be administered orally every second day from 6 years of age.
- Catch-up vaccination with TCV up to 15 years of age is recommended when feasible and supported by epidemiological data.
- Typhoid vaccination is recommended in response to confirmed outbreaks of typhoid fever and may be considered in humanitarian emergency settings depending on the risk assessment in the local setting.
- The potential need for revaccination with TCV is currently unclear. Revaccination is recommended every 3 years for ViPS, and every 3-7 years for Ty21a.
- Use of the live attenuated Ty21a vaccine during pregnancy should be avoided because of theoretical safety concerns about potential adverse effects.
Cholera vaccination schedule:
Appropriate case management, WaSH interventions, surveillance, and community mobilization remain the cornerstones of cholera control.

Vaccination should be implemented in relevant settings as part of comprehensive cholera control strategies or while other activities are being developed.
- WC vaccines 2 doses should be given 14 days apart to individuals ≥1 year of age.
- Revaccination is recommended where there is a continued risk of V. cholera infection. For WC vaccines revaccination is recommended after 3 years.
For WC-rBS vaccine:
Children age 2-5 years revaccination is recommended within 6 months. If less than 6 months have passed, 1 dose for revaccination. If more than 6 months have passed, the primary series of 3 doses should be repeated.
For those aged ≥6 years of age, if less than 2 years have passed, 1 dose for revaccination. If more than 2 years have passed, the primary series of 2 doses should be repeated.
- In cholera-endemic countries, vaccination of the entire population (throughout a country regardless of risk) is usually not warranted.
- Vaccination policies and strategies should be guided by an assessment of the risk of cholera and targeted to cholera hotspots.
- Strategies targeting specific age groups at higher risk of disease may be considered.
- For control of cholera, outbreaks vaccination should be considered to help prevent the spread to new areas.
- For vaccination campaigns, a single-dose strategy using WC vaccines could be considered in areas experiencing cholera outbreaks.

- During humanitarian emergencies with a risk of cholera, but without a current cholera outbreak, vaccination with OCV should be considered as an additional preparedness measure for outbreak prevention, depending on the local infrastructure (capacity to organize a vaccination campaign).
- Pregnant and lactating women and HIV infected individuals should be included in OCV campaigns since there are a high potential benefit and minimal risks.
Meningococcal vaccine schedule:
- Meningococcal meningitis and meningococcemia are life-threatening conditions. meningococcal infections are preventable via prior vaccination.

- Conjugate vaccines are preferred over polysaccharide vaccines due to their potential for herd protection and their increased immunogenicity, particularly in children <2 years of age.
- Both conjugate and polysaccharide vaccines are efficacious and safe when used in pregnant women.
- MenA conjugate vaccine (5μg) a 1-dose schedule is recommended at 9-18 months of age based on local programmatic and epidemiologic considerations. The vaccine should be administered by deep intramuscular injection, preferably in the anterolateral aspect of the thigh. There is no reason to expect interference when co-administered with other vaccines. The need for a booster dose has not been established.
- For monovalent MenC conjugate vaccine, one single intramuscular dose is recommended for children aged ≥12 months, teenagers and adults. Children 2-11 months require 2 doses administered at an interval of a least 2 months and a booster about 1 year after. If the primary series is interrupted, vaccination should be resumed without repeating the previous dose.
- Quadrivalent conjugate vaccines (A,C,W135,Y-D and A,C,W135,Y-CRM) should be administered as one single intramuscular dose to individuals ≥ 2 years. A,C,W135,Y-D is also licensed for children 9-23 months of age, and given as a 2-dose series, 3 months apart beginning at age 9 months. If the primary series is interrupted, vaccination should be resumed without repeating the previous dose.
- Meningococcal polysaccharide vaccines are less, or not, immunogenic in children under 2 years of age.
- Meningococcal polysaccharide vaccines can be used for those ≥ 2 years of age to control outbreaks in countries where limited economic resources or insufficient supply restrict the use of meningococcal conjugate vaccines.
- Polysaccharide vaccines should be administered to individuals ≥ 2 years old as one single dose. One booster 3-5 years after the primary dose may be given to persons considered to be a continued high risk of exposure, including some health workers. See position paper for details.
Hepatitis A vaccine schedule:
Hepatitis A vaccination is recommended for inclusion in the national immunization schedule for children ≥ 1 year if indicated on the basis of incidence of acute hepatitis A, change in the endemicity from high to intermediate, and consideration of cost-effectiveness

- In highly endemic countries almost all persons are asymptomatically infected with HAV in childhood, which effectively prevents clinical hepatitis A in adolescents and adults.
- In these countries, large-scale vaccination programs are not recommended.
- Countries with improving socioeconomic status may rapidly move from high to intermediate endemicity.
- In these countries, a relatively large proportion of the adult population is susceptible to HAV and large-scale hepatitis A vaccination is likely to be cost-effective and therefore is encouraged.
- For individual health benefit targeted vaccination of high-risk groups should be considered in low and very low endemicity settings.
- Those at increased risk of hepatitis A include travelers to areas of intermediate or high endemicity, those requiring life-long treatment with blood products, men who have sex with men, workers in contact with non-human primates, and injection drug users.
- In addition, patients with chronic liver disease are at increased risk for fulminant hepatitis A and should be vaccinated.
- Inactivated HAV vaccine is licensed for intramuscular administration in a 2-dose schedule with the first dose given at the age of 1 year or older. The interval between the first and second dose is flexible (from 6 months up to 4-5 years) but is usually 6-18 months.
- Countries may consider a 1-dose schedule as this option seems comparable in terms of effectiveness, and is less expensive and easier to implement.
- However, in individuals at substantial risk of contracting hepatitis A and in immunocompromised individuals, a 2-dose schedule is preferred.
- Live attenuated HAV vaccine is administered as a single subcutaneous dose to those older than 1 year of age.
- Severe allergy to components included in the live attenuated hepatitis A vaccine is a contraindication to their use.
- As a rule, live vaccines should not be used in pregnancy or in severely immunocompromised patients.
- There is no information available on co-administration of live attenuated hepatitis A vaccines with other routinely used vaccines.
- Vaccination against hepatitis A should be part of a comprehensive plan for the prevention and control of viral hepatitis, including measures to improve hygiene and sanitation and measures for outbreak control.
Varicella vaccination schedule:
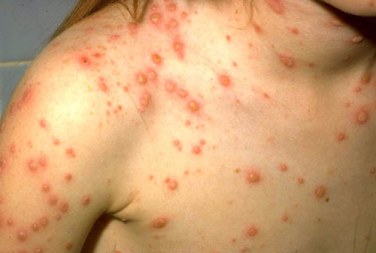
Countries, where varicella is an important public health burden, could consider introducing varicella vaccination in the routine childhood immunization program.
However, resources should be sufficient to ensure reaching and sustaining vaccine coverage ≥ 80%. Decisionmaking on childhood varicella vaccination should also include consideration of the possible impact on herpes zoster.
- Depending on the goal of the vaccination programme, 1-2 doses should be given with the first dose administered at 12-18 months of age. The minimum interval between doses should be as recommended by the manufacturer, ranging from 4 weeks to 3 months.
- Varicella vaccination is contraindicated during pregnancy and pregnancy should be delayed for 4 weeks after vaccination.
- Termination of pregnancy is not indicated if vaccination was carried out inadvertently during pregnancy.
- Varicella vaccine can be administered concomitantly with other vaccines. Unless given together with other live viral vaccines (measles, MR, MMR), it should be administered at a minimum interval of 28 days.
- Countries should consider vaccination of potentially susceptible health-care workers (i.e. unvaccinated and with no history of varicella) with 2 doses of varicella vaccine.